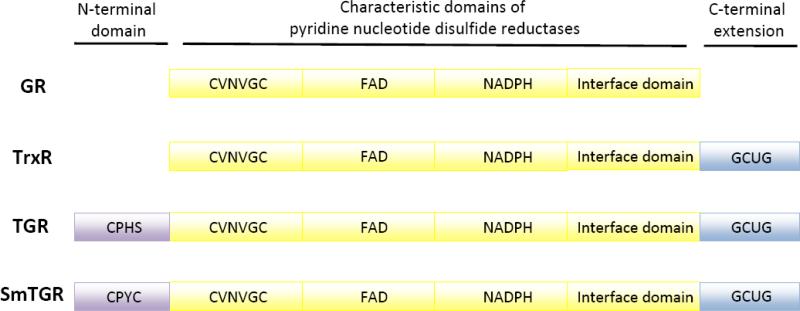
Figure 2.
Comparison of the domain structure of pyridine nucleotide disulfide oxidoreductases. Glutathione reductase (GR), thioredoxin reductase (TrxR) and thioredoxin glutathione reductase (TGR) all contain a common domain characteristic of pyridine nucleotide disulfide oxidoreductases (yellow) with N-terminal redox active cysteines (CVNVGC), FAD- and NADPH-binding pockets and a interface domain that is involved in protein dimerization. TrxR and TGR proteins both contain a C-terminal extension (blue) with redox active cysteine and selenocysteine (U) (or cysteine). TGR proteins have an additional N-terminal domain with sequence and structural similarities to glutaredoxin (purple). This domain has a redox active cysteine pair (CPYC) as in Schistosoma mansoni TGR or single cysteine (CPHS) as in human TGR.