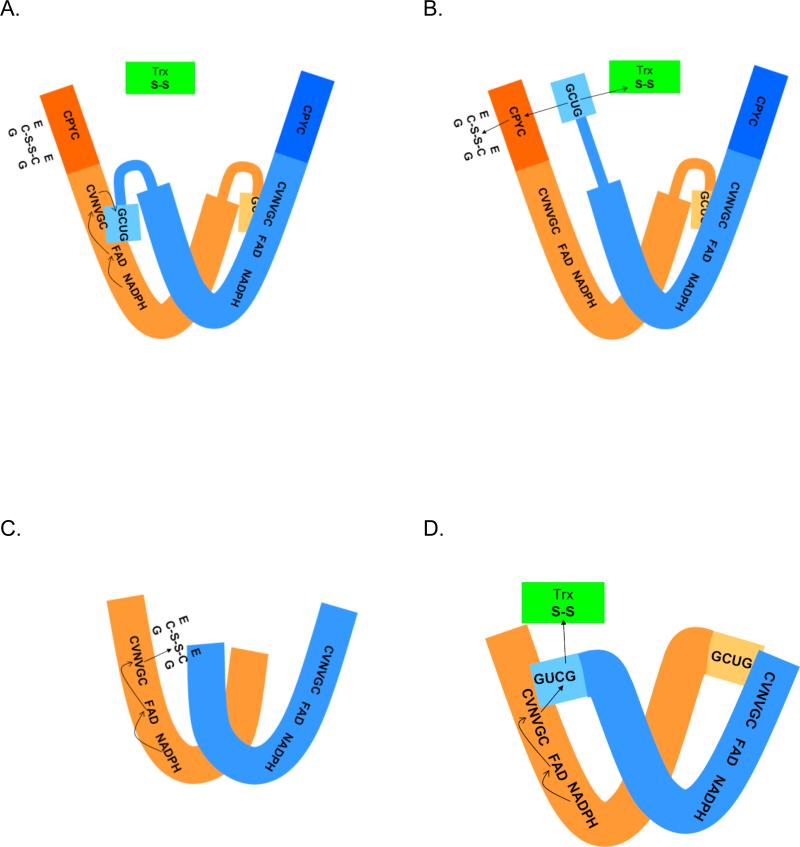
Figure 3.
Comparison of the proposed reaction mechanisms of pyridine nucleotide disulfide oxidoreductases. The active forms of all proteins are dimmers of identical subunits. (A) and (B) Schistosoma mansoni thioredoxin glutathione reductase. In (A) reducing equivalents are shown being transferred from NADPH to the FAD and then to the adjacent cysteine pair in the sequence (CVNVGC). This reduced cysteine pair then transfers the hydride to the C-terminal cysteineselenocysteine in the other peptide chain. (B) Reduction of the C-terminal cysteine-selenocysteine pair produces a structural change in the flexible C-terminal arm allowing it to be repositioned to either react with the glutaredoxin domain active site (CPYC) or directly with oxidized thioredoxin (and potentially other substrates). The reduced glutaredoxin domain can then interact with its substrates, glutathione disulfide (shown) or other substrates such as glutathionylated peptides. In (C), electrons from NADPH pass to the flavin and then to the redox active cysteine pair (CVNVGC) and subsequently to glutathione disulfide in glutathione reductase proteins. In (D), electrons from NADPH pass to the flavin, then to the redox active cysteine pair (CVNVGC) and subsequently to the C-terminal cysteine-selenocysteine couple in the other subunit in thioredoxin reductases. The reduced C-terminal active site can then transfer reducing equivalents to substrates (e.g., oxidized thioredoxin).