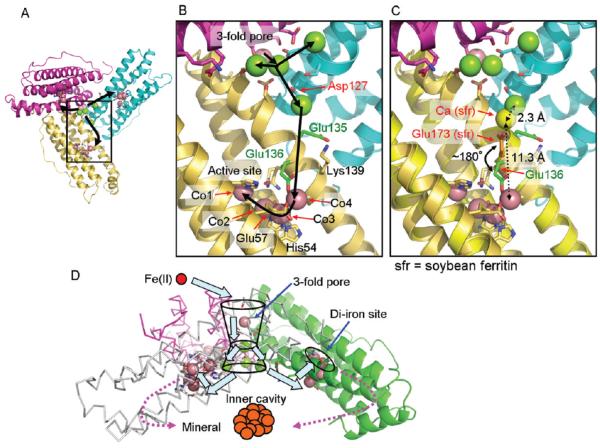
Figure 4.
Co(II)- and Mg(II)-binding sites in the ion channels, at the inner cavity surface, and around the actives sites of ferritin–protein cages show the path from pores to active sites and indicate how eight ion channels can provide Fe(II) substrate to 24 oxidoreductase sites. (A) The inner cavity surface of three subunits at the ion channel pore; black arrows show the proposed Fe(II) path from the external pores to three active sites. (B) The same view enlarged to show a single subunit. Green, Mg(II) ions; magenta, Co(II) ions. (C) Superposition of soybean ferritin (PDB ID 3A68) on frog M ferritin to show a plausible alternative configuration of Glu136 (green stick) in frog M ferritin to assist in Fe(II) transfer from Asp127 in the ion channels to the active sites. Yellow stick, Glu173 in soybean, which corresponds to Glu136 in frog M ferritin; orange stick, Glu136; yellow sphere, Ca bound to Glu173. (D) Schematic overview of the trajectory of iron as Fe(II) enters the ferritin–protein nanocage through three-fold ion channels, moves to the active sites, and, as Fe(III)O complexes, leaves the catalytic sites to traverse the postoxidation channels for mineral nucleation and exit into the mineralization cavity.