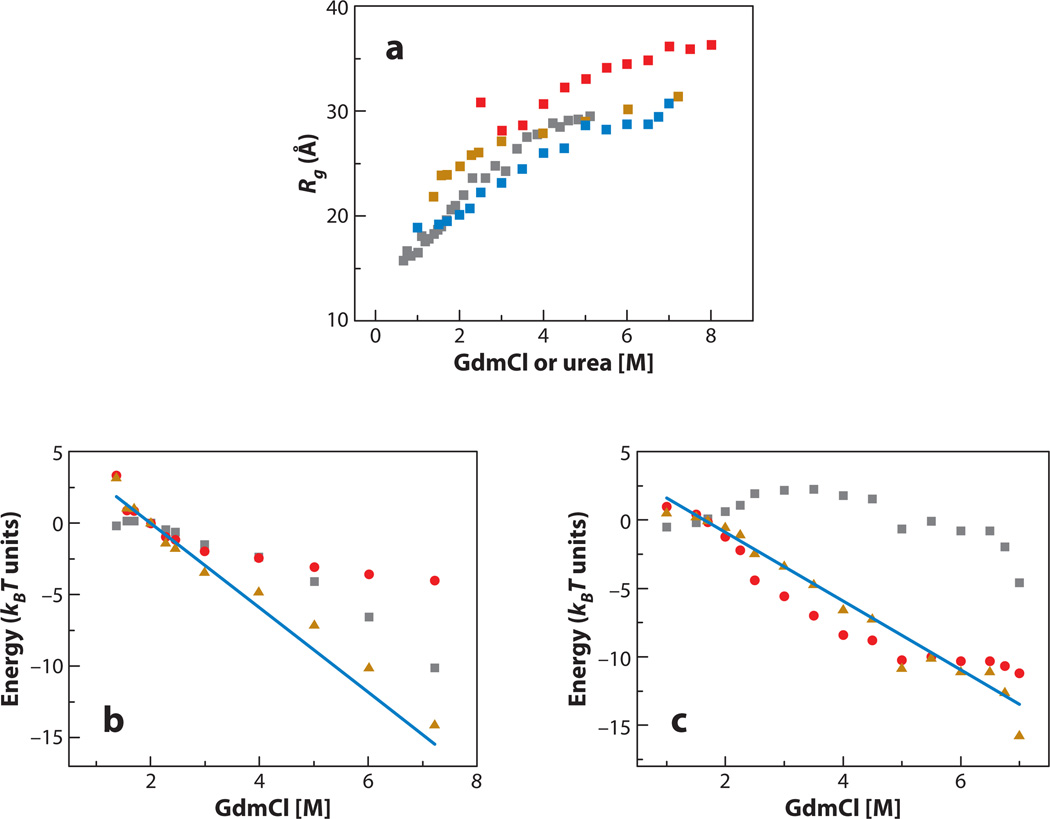
Figure 5.
Properties of denatured proteins as a function of denaturant concentration, calculated from the coil-globule transition theory (79). (a) The radius of gyration of the cold shock protein (gold, based on data from 80), protein L (blue, based on data from 78), immunity protein 9 (red, based on data from 65) and barstar (gray, based on data from 82). (b) Expansion difference free energy, ΔGC→U, of denatured cold shock protein (gold triangles) compared with its m value (blue line). Also shown are the expansion enthalpy (gray squares) and conformational entropy (red circles). (c) Expansion difference free energy, ΔGC→U, of denatured protein L (gold triangles) compared with its m value (blue line). Also shown are the expansion enthalpy (gray squares) and conformational entropy (red circles). Clearly, conformational entropy contributes significantly to the expansion free energy and hence also to the free energy of unfolding. GdmCl, guanidinium chloride.