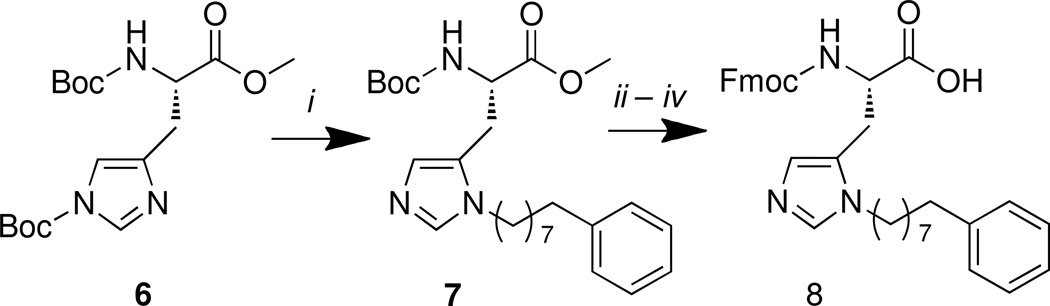
Scheme 1.
Reagents and conditions: (i) Ph(CH2)7CH2OH (1.1 equiv.), Tf2O (1.1 equiv), DIPEA (1.1 equiv.), CH2Cl2, −75 °C – rt, 16 – 18 h, 60% yield; (ii) LiOH•H2O (2.0 equiv.), THF, H2O (v/v 4:1), 0 °C – rt, 1 h; (iii) 4 M HCl in dioxane (10 equiv.), rt, 1 h; (iv) Fmoc-OSu (1.5 equiv.), NaHCO3 (4.0 equiv.), THF – H2O (v/v 1:1), rt, 12 h (82% over three steps).