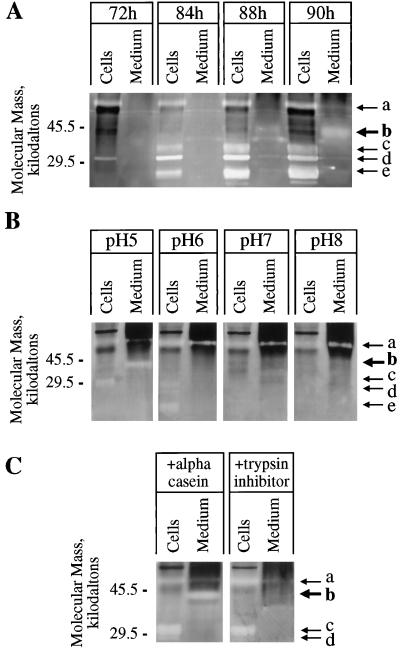
Figure 9.
Timing of expression and characteristics of proteases expressed by differentiating TEs. A, Intracellular proteins (Cells) and proteins concentrated from media (Medium) of the same culture at the indicated times after culture initiation were assayed on protease activity gels as described in Methods. After development, protease activities are recognized as clear bands resulting from hydrolysis of the gelatin substrate. At the time of harvest, the percentages of dead TEs were 0%, 20%, 49%, and 78% for the 72-, 84-, 88-, and 90-h cultures, respectively. Several intracellular protease activities can be seen (arrows a, c, d, and e), similar to the findings of Beers and Freeman (1997) and Ye and Droste (1996). Protease activity is visible at approximately 40 kD (arrow b) in media after 84 h. The exact time during development that protease secretion commences cannot be determined directly from this technique, and accumulation of detectable protease activity in the medium may significantly lag behind the onset of secretion. Approximately 0.015 μg of medium protein and 0.5 μg of intracellular protein was loaded per sample. B, Aliquots of the same preparations of intracellular proteins (90-h culture) and medium proteins (88-h culture) were run on the same protease activity gel. After fractionation the gel was sliced into four pieces, and each piece was incubated in an activity buffer with the indicated pH overnight (see Methods) before development. The 40-kD activity in medium proteins (arrow b) is detected only at pH 5. C, Aliquots of the same preparations of intracellular proteins (90-h culture) and medium proteins (88.5-h culture) were run on the same protease activity gel. The gel was divided in half, and one-half was immersed in ice-cold activity buffer containing 10 mg/mL soybean trypsin inhibitor (21 kD) and the other half was immersed in ice-cold activity buffer containing 10 mg/mL dephosphorylated α-casein (23 kD) for 45 min to allow the proteins to diffuse into the gels. Gels were then incubated at room temperature overnight before development. α-Casein has no protease inhibitory property, so it was used as a control for increasing background staining attributable to protein infusion into the gel. The soybean-trypsin-infused gel does not show the 40-kD activity in the medium, whereas the α-casein-infused gel does show the 40-kD activity (arrow b), indicating that the activity was not simply obscured by the infused proteins, but was specifically inhibited by soybean trypsin inhibitor. {/ANNT;;;left;top}