Abstract
Nitrogen-doped TiO2 (N-TiO2) nanoparticles were prepared by calcining the anatase TiO2 nanoparticles under ammonia atmosphere. The N-TiO2 showed higher absorbance in the visible region than the pure TiO2. The cytotoxicity and visible-light-induced phototoxicity of the pure- and N-TiO2 were examined for three types of cancer cell lines. No significant cytotoxicity was detected. However, the visible-light-induced photokilling effects on cells were observed. The survival fraction of the cells decreased with the increased incubation concentration of the nanoparticles. The cancer cells incubated with N-TiO2 were killed more effectively than that with the pure TiO2. The reactive oxygen species was found to play an important role on the photokilling effect for cells. Furthermore, the intracellular distributions of N-TiO2 nanoparticles were examined by laser scanning confocal microscopy. The co-localization of N-TiO2 nanoparticles with nuclei or Golgi complexes was observed. The aberrant nuclear morphologies such as micronuclei were detected after the N-TiO2-treated cells were irradiated by the visible light.
Introduction
Semiconductor titanium dioxide (TiO2) has been widely studied as a photocatalyst for its high chemical stability, excellent oxidation capability, good photocatalytic activity, and low toxicity [1-4]. Under the irradiation of ultraviolet (UV) light with the wavelength shorter than 387 nm (corresponding to 3.2 eV for the band gap of anatase TiO2), the electrons in the valence band of TiO2 can be excited to the conduction band, thus creating the pairs of photo-induced electron and hole. Then, the photo-induced electrons and holes can lead to the formation of various reactive oxygen species (ROS), which could kill bacteria, viruses, and cancer cells [5-10].
In recent years, TiO2 attracted more attention as a photosensitizer in the field of photodynamic therapy (PDT) due to its low toxicity and high photostability [2,3]. However, TiO2 can be activated by UV light only, which hinders its applications. Improvement of the optical absorption of TiO2 in the visible region by dye-adsorbed [11,12] or doping [13,14] methods will facilitate the practical application of TiO2 as a photosensitizer for PDT. When using dye-adsorbed method, the dyes such as hypocrellin B [11] and chlorine e6 [12] themselves are well-known PDT sensitizers and will have influence on the PDT efficiency of TiO2. For doping method, anionic species are preferred for the doping rather than cationic metals which have a thermal instability and an increase of the recombination centers of carriers [14]. In addition, cationic metals themselves always present cytotoxicity. Therefore, anionic species doping, especially nitrogen doping, is mostly adopted to improve the absorption of TiO2 in the visible region.
In the present work, the nitrogen-doped TiO2 (N-TiO2) nanoparticles were used as the photosensitizer to test its photokilling efficiency for three types of cancer cell lines. The N-TiO2 nanoparticles were prepared by calcining pure anatase TiO2 nanoparticles under ammonia atmosphere, which was an inexpensive method and easy to operate. The produced N-TiO2 nanoparticles have high stability and effective photocatalytic activity. Their absorption in the visible region was improved and their photokilling efficiency of cells under visible-light irradiation was compared with that of the pure TiO2. The intracellular distributions of these nanoparticles were measured by the laser scanning confocal microscopy (LSCM). The mechanisms of the photokilling effect were discussed.
Methods
Preparation and characterization of N-TiO2 nanoparticles
The anatase TiO2 nanoparticles (Sigma-Aldrich, St. Louis, MO, USA; particle size <25 nm) were calcined under ammonia atmosphere with various calcination parameters, such as temperature, gas flow rate, and calcination time, and then cooled down in nitrogen flow to the room temperature. Three N-TiO2 samples prepared with different calcination parameters were used in this work. Together with the pure TiO2, they are denoted as listed in Table 1. The crystalline phases of these samples were determined by Raman spectra (LABRAM-1B; HORIBA, Jobin Yvon, Kyoto, Japan). To evaluate their absorptions in the visible region, the ultraviolet-visible (UV/Vis) diffuse reflectance absorption spectra of these samples were measured with a Jasco V550 UV/Vis spectrophotometer (Jasco, Inc., Tokyo, Japan)
Table 1.
Calcination parameters and the resulted crystalline phases of the TiO2 nanoparticles
| Samples | Calcination parameters | Crystalline phases | ||
|---|---|---|---|---|
| Temperature (°C) | Ammonia gas flow rate (L/min) | Time (min) | ||
| Pure | - | - | - | Anatase |
| N-550-1 | 550 | 3.5 | 20 | Anatase |
| N-550-2 | 550 | 7 | 10 | Anatase |
| N-600-1 | 600 | 3.5 | 20 | Rutile and anatase |
Pure- and N-TiO2 nanoparticles were dispersed in Dulbecco's modified Eagle's medium with high glucose (DMEM-H), respectively, at various concentrations between 50 and 200 μg/mL. To avoid aggregation, these suspensions were ultrasonically processed for 15 min before using.
Cell culture
The human cervical carcinoma cells (HeLa), human hepatocellular carcinoma cells (QGY), or human nasopharyngeal carcinoma cells (KB) procured from the Cell Bank of Shanghai Science Academy (Shanghai, China) were grown in 96-well plates or Petri dishes in DMEM-H solution supplemented with 10% fetal calf serum in a fully humidified incubator at 37°C with 5% CO2 for 24 h. Then, the culture medium was replaced by TiO2-containing medium and the cells were incubated for 2 h in the dark. After the TiO2 nanoparticles deposited and adhered to the cells, the medium was changed to the TiO2-free DMEM-H solution supplemented with 10% fetal calf serum for further study.
Measurements of photokilling effect and cytotoxicity
To examine the photokilling effect, the cells were irradiated with the visible light from a 150-W Xe lamp (Shanghai Aojia Electronics Co. Ltd., Shanghai, China). Two pieces of quartz lens were used to obtain a concentrated parallel light beam. An IR cutoff filter was set in the light path to avoid the hyperthermia effect. A 400-nm longpass filter was used to cut off the UV light. The visible-light power density at the liquid surface in cell wells was 12 mW/cm2 as measured by a power meter (PM10V1; Coherent, Santa Clara, CA, USA). After irradiation with this visible light for 4 h, cells were incubated in the dark for another 24 h until further analysis were conducted. The cytotoxicity examinations were carried out with the same procedure as the photokilling effect examinations but without the light irradiation, i.e., the TiO2-treated cells were incubated in the dark for 28 h.
The cell viability assays were conducted by a modified MTT method using WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H tetrazolium, monosodium salt] (Beyotime, Jiangsu, China). Each well containing 100 μL culture medium was added with 10 μL of the WST-8 reagent solution, and the cells were then incubated at 37°C with 5% CO2 for 2 h. Subsequently, the absorbance was measured at 450 nm using a microplate reader (Bio-Tek Synergy™ HT; Bio-Tek® Instruments, Inc., Winooski, VT, USA). The untreated cells were used as the control groups. The surviving fraction represents the ratio of the viable TiO2-treated cells relative to that of the control groups. It should be noted that the TiO2-containing DMEM-H solution will affect the absorbance value at 450 nm. Therefore, when measuring the cell viability, the absorbance values were measured as a reference before the WST-8 dyes were added. Each experiment was performed in triplicate and repeated three times.
Confocal laser scanning microscopy
The cells grown in Petri dishes were incubated with 50 μg/mL TiO2 in DMEM-H for 10 h before the LSCM observation (Olympus, FV-300, IX71; Olympus, Tokyo, Japan). Hoechst 33342 (Beyotime) and BODIPY FL C5-ceramide complexed to BSA (Molecular Probes; Invitrogen Corporation, Eugene, OR, USA) were used as the indicators for nucleus and Golgi complex, respectively. Hoechst 33342 (0.5 μg/mL) or Golgi complex marker (5 μM) was added into the growth medium for 15 to 30 min to stain the nuclei or Golgi complexes, respectively.
The reflection images of the intracellular TiO2 nanoparticles and the fluorescence images of nuclei (or Golgi complexes) were simultaneously obtained by the LSCM in two channels with no filter for the reflecting light and a 585 to 640-nm bandpass filter for the fluorescence. A 488-nm continuous-wave (CW) Ar+ laser (Melles Griot, Carlsbad, CA, USA) or a 405-nm CW semiconductor laser (Coherent) was used as the excitation source. A 60 × water objective was used to focus the laser beam to a spot of about 1 μm in diameter. The differential interference contrast (DIC) micrographs to exhibit the cell morphology were acquired in a transmission channel simultaneously. The three-dimensional (3D) distributions of TiO2 nanoparticles and nuclei (or Golgi complexes) were obtained using the z-scan mode of the microscope.
Results and discussion
Raman spectra of TiO2 nanoparticles
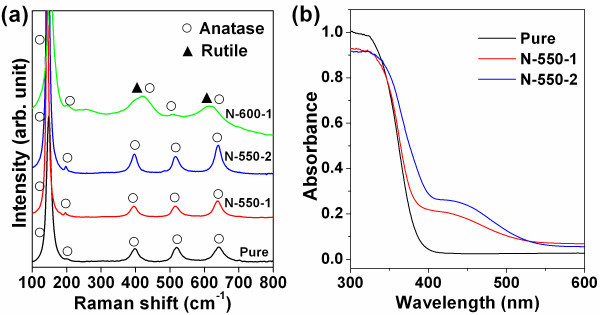
As shown in Table 1 and Figure 1a, the N-TiO2 samples N-550-1 and N-550-2 with the calcination temperature of 550°C, as well as the pure TiO2, exhibited a similar feature with five Raman peaks around 143, 197, 395, 514, and 640 cm-1, corresponding to the Raman fundamental modes of the anatase phase [15,16]. The Raman peaks for rutile phase [16] around 238, 420, and 614 cm-1 appeared when the calcination temperature was 600°C as shown in the spectrum of the sample N-600-1. It can be concluded that the phase of the TiO2 nanoparticles would transform from anatase to rutile when the calcination temperature increased to 600°C. Such a phase transformation will result in a decrease of the photocatalytic ability for TiO2 powders [17,18]. Therefore, we only used samples N-550-1 and N-550-2 for further studies.
Figure 1.
Raman and UV/Vis diffuse reflectance spectra of the nanoparticle samples. (a) Raman spectra of the pure and the three N-TiO2 nanoparticle samples. (b) Diffuse reflectance absorption spectra of samples pure, N-550-1, and N-550-2. Sample N-550-2 exhibited the highest absorbance in the visible region.
Absorption spectra of TiO2 nanoparticles
Figure 1b shows the absorption spectra of the samples N-550-1 and N-550-2 and pure TiO2. Compared to the pure TiO2, the absorbances of N-550-1 and N-550-2 are higher in the visible region. However, the sample N-550-2 has the higher absorbance than N-550-1 in the region of 400 to 500 nm. Since N-550-1 and N-550-2 were calcinated at the same temperature and with the same amount of ammonia (flow rate times time), it seems that higher ammonia flow rate (N-550-2) could cause more absorption in the visible, which was expected to have higher photokilling efficiency of cells.
Cytotoxicity and photokilling effect
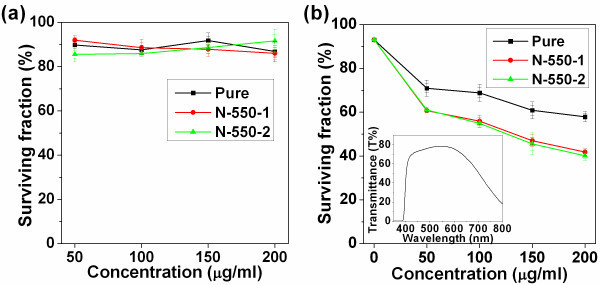
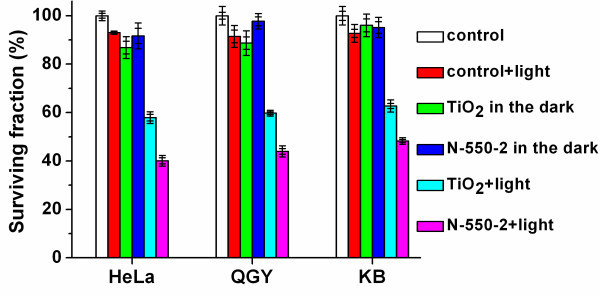
To evaluate the cytotoxicity of pure- and N-TiO2 nanoparticles, the TiO2-treated cells were further incubated in the dark for 28 h and the cell viability assays were then conducted. As shown in Figure 2a, all the surviving fractions of the treated HeLa cells were on the average values greater than 85% (with the concentration from 50 to 200 μg/mL). As shown in Figure 3, all the surviving fractions of the treated QGY or KB cells with the pure- or N-TiO2 concentration of 200 μg/mL in the dark were greater than 85%. These results indicated that the cytotoxicities of pure- and N-TiO2 nanoparticles were quite low. The cytotoxicities of these nanoparticles were quite similar, and there was no significant influence of the concentration on the cytotoxicity. Pure TiO2 is biocompatible with primary and cancer cells [4]. Nitrogen is an essential element of many biological molecules, such as proteins and nucleic acids. So, nitrogen is not toxic if it does not exceed the normal levels. It could be understood that a small amount of nitrogen doping would not lead to more cytotoxicity than pure TiO2.
Figure 2.
Surviving fraction of treated and untreated HeLa cells. (a) Surviving fraction of HeLa cells as a function of the concentration of TiO2 nanoparticles. HeLa cells were treated with 50, 100, 150, and 200 μg/mL TiO2, respectively, in the dark. The surviving fraction of untreated cells (control group) was set as 100%. (b) The photokilling effects of pure and N-TiO2 with different concentrations under visible irradiation. The inset is the transmittance spectrum of the combination of a 400 nm longpass filter and an IR cutoff filter used to acquire the visible-light irradiation from a Xe lamp.
Figure 3.
The cytotoxicities and the photokilling effects of pure TiO2 and N-550-2 samples. With the concentration of 200 μg/mL on HeLa, QGY, and KB cells. The control groups were also shown for comparison.
The photokilling effects were measured as described in the experimental section. The surviving fractions of HeLa cells under visible-light irradiations for 4 h in dependence on the concentrations of pure- and N-TiO2 nanoparticles were shown in Figure 2b. As demonstrated in Figure 2b, the visible light showed very little photokilling effect on HeLa cells in the absence of any TiO2 (pure or N-doped) (at the 0 concentration). The surviving fractions (compared to the control cells without irradiation) were around 93%, which might be caused by the light irradiation, the fluctuant temperature during irradiation, and the experimental procedures. The spectrum of the light irradiated on cells (with filters) is also shown in the figure as an inset. It should be noted according to the spectrum in Figure 1b that the pure TiO2 nanoparticles still has some absorption around 400 nm though the band gap of TiO2 was reported to be 3.2 eV (corresponding to a wavelength of 387 nm). Therefore, pure TiO2 exhibited some photokilling effect under visible-light irradiation as shown in Figure 2b. However, the cells treated with N-TiO2 were killed more effectively than that with pure TiO2. The photokilling effects of samples N-550-1 and N-550-2 were quite similar although their absorption spectra showed some difference. It is also demonstrated in Figure 2b that the survival fractions decreased with the increasing concentrations of the TiO2 samples. It decreased to 40% for the cells treated with sample N-550-2 at a concentration of 200 μg/mL.
The photokilling effects of sample N-550-2 at a concentration of 200 μg/mL on QGY and KB cells were also measured as shown in Figure 3. Similar with the photokilling effect on HeLa cells, the QGY and KB cells treated with N-550-2 were also killed more effectively than that with pure TiO2 under the visible-light irradiation. The results revealed that the N-TiO2 might be applied to different cancers as a photosensitizer for PDT.
ROS influence on the photokilling effect
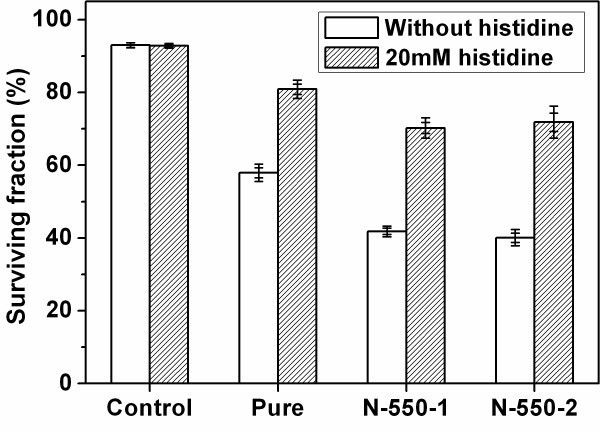
The mechanism of the photokilling effect for cancer cells caused by TiO2 nanoparticles is very complex. It has been identified that UV-photoexcited TiO2 in aqueous solution will result in formation of various ROS, such as hydroxyl radicals (·OH), hydrogen peroxide (H2O2), superoxide radicals (·O2-) and singlet oxygen (1O2) [19,20]. The ROS will attack the cancer cells and finally lead to the cell death. In order to study the function of ROS on the photokilling effect, the L-histidine, a quencher for both 1O2 and ·OH [21-23], was added into the 96-well plates (20 mM) 30 min before the cells were irradiated by light. In the presence of 20 mM L-histidine, all the surviving fractions of the cells treated with pure- and N-TiO2 at a concentration of 200 μg/mL increased evidently as shown in Figure 4. These results are similar to the previous report for UV-photoexcited TiO2 [14]. It can be concluded that the ROS plays an important role on the photokilling effect, although we cannot tell which one played the main role. Further research is needed to figure out all the ROS influences.
Figure 4.
Changes in the surviving fractions of the TiO2-treated HeLa cells with histidine. The concentration of the three TiO2 samples is 200 μg/mL and L-histidine is 20 mM.
Distribution of TiO2 in cells
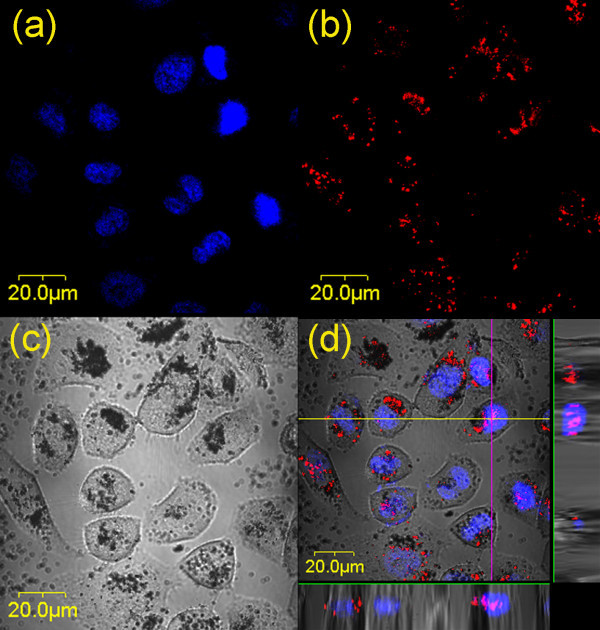
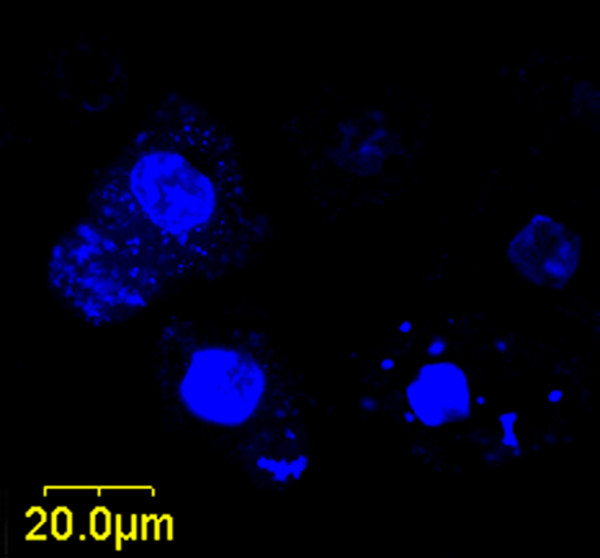
As is well-known, light-excited TiO2 generates the electron-hole (e-/h+) pairs. The photogenerated carriers migrate to the particle surface and participate in various redox reactions there. Hence, the direct damage induced by photokilling effect would only occur at the sites of TiO2 particles. Therefore, it is of importance to know if the TiO2 nanoparticles were internalized into cells and how their intracellular distributions were. To find out the subcellular distribution of TiO2 nanoparticles, the TiO2-treated HeLa cells were stained with fluorescence indicators for Golgi complex and nucleus, respectively. Surprisingly, some TiO2 nanoparticles were found inside the nuclei as shown in Figure 5, where the HeLa cells were treated with (N-550-2, 50 μg/mL) and stained with nuclear indicator. When these N-TiO2-treated cells were irradiated by light from the Xe lamp with a 400-nm longpass filter (12 mW/cm2) for 4 h, some micronuclei were observed as shown in Figure 6. Since the TiO2 nanoparticles had entered into the nuclei of cells, the photoactivation effect could occur directly inside the nuclei, which might cause chromosomal damage or nucleus aberration. Micronuclei are usually formed from a chromosome or a fragment of a chromosome not incorporated into one of the daughter nuclei during cell division. This is an evidence of the direct damage to the nucleus resulted from the photoexcited N-TiO2 nanoparticles.
Figure 5.
Micrographs of the distributions of nuclei and TiO2 nanoparticles in HeLa cells. (a) the distribution of nuclei (blue), (b) the distribution of TiO2 nanoparticles (red), (c) DIC micrograph, and (d) the merged image of (a), (b), and (c), in which the violet color denotes the co-localization of TiO2 nanoparticles with nuclei. The images displayed at the bottom and right side of (d) were the X-Z and Y-Z profiles measured along the lines marked in the main image, showing the 3D distributions of TiO2 nanoparticles and nuclei.
Figure 6.
Micrograph of the micronuclei of the HeLa cells. Cultured with 50 μg/mL sample N-550-2 for 10 h and irradiated by a Xe lamp with a 400-nm longpass filter (12 mW/cm2) for 4 h. The micronuclei were observed.
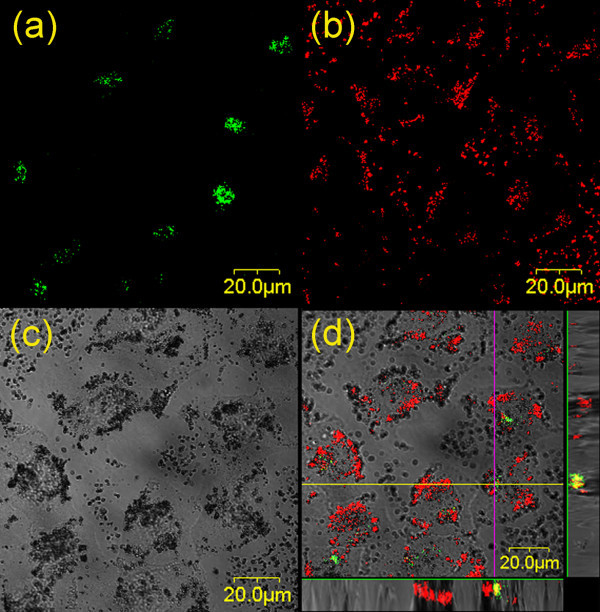
Figure 7 is the confocal micrographs to show the distributions of Golgi complexes (fluorescence image) and TiO2 nanoparticles (reflection image) in HeLa cells. As shown in the merged image in Figure 7d, the TiO2 particles were not only found on the cell membrane but also in the cytoplasm. Some TiO2 nanoparticles aggregated around or in Golgi complexes. The co-localizations of TiO2 with Golgi complexes (yellow color) were observed. The cell viability might be influenced by the localization of TiO2 in Golgi complexes or other cell organelles, although there is no direct evidence found in this work.
Figure 7.
Micrographs of the distributions of Golgi complexes and TiO2 nanoparticles in HeLa cells. (a) The distribution of Golgi complexes (green), (b) the distribution of TiO2 nanoparticles (red), (c) differential interference contrast (DIC) micrograph, and (d) the merged image of (a), (b), and (c), in which the yellow color denotes the co-localization of TiO2 nanoparticles with Golgi bodies. The images displayed at the bottom and right side of (d) were the X-Z and Y-Z profiles measured along the lines marked in the main image, showing the 3D distributions of TiO2 and Golgi bodies.
Conclusions
In the present work, N-TiO2 nanoparticles were prepared by calcination under ammonia atmosphere, which is an easily operative method and can achieve the product fruitfully. All the cytotoxicities of the pure- or N-TiO2 nanoparticles were quite low. The N-TiO2 samples showed higher absorbance and better photokilling effect than the pure TiO2 in the visible region. Therefore, the N-TiO2 has a higher potential as a photosensitizer for PDT of cancers.
TiO2 is nonfluorescent and cannot be detected by fluorescence imaging. However, it can be monitored by the reflection imaging, which makes it convenient to record simultaneously with the fluorescence image using a LSCM. Co-localization of N-TiO2 nanoparticles with nuclei was observed. After visible-light irradiation, some micronuclei were detected as a sign of the nucleus aberration. Furthermore, ROS was found to play an important role on the photokilling effect for cells. However, the mechanisms for the photokilling effect on cancer cells should be investigated in details further.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ZL carried out the experiments and drafted the manuscript. LM designed the project, participated in the confocal microscopy imaging, and wrote the manuscript. PW supervised the work and participated in the discussion of the results and in revising the manuscript. JC participated in the discussion of the results. All authors read and approved the final manuscript.
Contributor Information
Zheng Li, Email: 081021011@fudan.edu.cn.
Lan Mi, Email: lanmi@fudan.edu.cn.
Pei-Nan Wang, Email: pnwang@fudan.edu.cn.
Ji-Yao Chen, Email: jychen@fudan.edu.cn.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (61008055, 11074053), the Ph.D. Programs Foundation of Ministry of Education of China (20100071120029), and the Shanghai Educational Development Foundation (2008CG03).
References
- Szacilowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G. Bioinorganic photochemistry: Frontiers and mechanisms. Chem Rev. 2005;105:2647–2694. doi: 10.1021/cr030707e. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, van Ravenzwaay B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol. 2008;82:151–157. doi: 10.1007/s00204-007-0253-y. [DOI] [PubMed] [Google Scholar]
- Carbone R, Marangi I, Zanardi A, Giorgetti L, Chierici E, Berlanda G, Podestà A, Fiorentini F, Bongiorno G, Piseri P, Pelicci PG, Milani P. Biocompatibility of cluster-assembled nanostructured TiO2 with primary and cancer cells. Biomaterials. 2006;27:3221–3229. doi: 10.1016/j.biomaterials.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Adams LK, Lyon DY, Alvarez PJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Thevenot P, Cho J, Wavhal D, Timmons RB, Tang LP. Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomed-Nanotechnol. 2008;4:226–236. doi: 10.1016/j.nano.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet L, Lyon DY, Hotze EM, Alvarez PJJ, Wiesner MR. Comparative photoactivity and antibacterial properties of C60 fullerenes and titanium dioxide nanoparticles. Environ Sci Technol. 2009;43:4355–4360. doi: 10.1021/es803093t. [DOI] [PubMed] [Google Scholar]
- Choi O, Hu ZQ. Role of reactive oxygen species in determining nitrification inhibition by metallic/oxide nanoparticles. J Environ Eng-Asce. 2009;135:1365–1370. doi: 10.1061/(ASCE)EE.1943-7870.0000103. [DOI] [Google Scholar]
- Lagopati N, Kitsiou PV, Kontos AI, Venieratos P, Kotsopoulou E, Kontos AG, Dionysiou DD, Pispas S, Tsilibary EC, Falaras P. Photo-induced treatment of breast epithelial cancer cells using nanostructured titanium dioxide solution. J Photoch Photobio A. 2010;214:215–223. doi: 10.1016/j.jphotochem.2010.06.031. [DOI] [Google Scholar]
- Zhang DQ, Li GS, Yu JC. Inorganic materials for photocatalytic water disinfection. J Mater Chem. 2010;20:4529–4536. doi: 10.1039/b925342d. [DOI] [Google Scholar]
- Xu SJ, Shen JQ, Chen S, Zhang MH, Shen T. Active oxygen species (1O2, O2·-) generation in the system of TiO2 colloid sensitized by hypocrellin B. J Photoch Photobio B. 2002;67:64–70. doi: 10.1016/S1011-1344(02)00263-4. [DOI] [PubMed] [Google Scholar]
- Tokuoka Y, Yamada M, Kawashima N, Miyasaka T. Anticancer effect of dye-sensitized TiO2 nanocrystals by polychromatic visible light irradiation. Chem Lett. 2006;35:496–497. doi: 10.1246/cl.2006.496. [DOI] [Google Scholar]
- Janczyk A, Wolnicka-Głubisz A, Urbanska K, Stochel G, Macyk W. Photocytotoxicity of platinum(IV)-chloride surface modified TiO2 irradiated with visible light against murine macrophages. J Photoch Photobio B. 2008;92:54–58. doi: 10.1016/j.jphotobiol.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Janczyk A, Wolnicka-Głubisz A, Urbanska K, Kisch H, Stochel G, Macyk W. Photodynamic activity of platinum(IV) chloride surface-modified TiO2 irradiated with visible light. Free Radical Bio Med. 2008;44:1120–1130. doi: 10.1016/j.freeradbiomed.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Chen XB, Lou YB, Samia ACS, Burda C, Gole JL. Formation of oxynitride as the photocatalytic enhancing site in nitrogen-doped titania nanocatalysts: Comparison to a commercial nanopowder. Adv Funct Mater. 2005;15:41–49. doi: 10.1002/adfm.200400184. [DOI] [Google Scholar]
- Wang H, Wu Y, Xu BQ. Preparation and characterization of nanosized anatase TiO2 cuboids for photocatalysis. Appl Catal B. 2005;59:139–146. doi: 10.1016/j.apcatb.2005.02.001. [DOI] [Google Scholar]
- Mi L, Xu P, Wang PN. Experimental study on the bandgap narrowings of TiO2 films calcined under N2 or NH3 atmosphere. Appl Surf Sci. 2008;255:2574–2580. doi: 10.1016/j.apsusc.2008.07.150. [DOI] [Google Scholar]
- Wantala K, Laokiat L, Khemthong P, Grisdanurak N, Fukaya K. Calcination temperature effect on solvothermal Fe-TiO2 and its performance under visible light irradiation. J Taiwan Inst Chem E. 2010;41:612–616. doi: 10.1016/j.jtice.2010.01.008. [DOI] [Google Scholar]
- Daimon T, Nosaka Y. Formation and behavior of singlet molecular oxygen in TiO2 photocatalysis studied by detection of near-infrared phosphorescence. J Phys Chem C. 2007;111:4420–4424. doi: 10.1021/jp070028y. [DOI] [Google Scholar]
- Tachikawa T, Majima T. Single-molecule detection of reactive oxygen species: application to photocatalytic reactions. J Fluoresc. 2007;17:727–738. doi: 10.1007/s10895-007-0181-5. [DOI] [PubMed] [Google Scholar]
- Wade AM, Tucker HN. Antioxidant characteristics of L-histidine. J Nutr Biochem. 1998;9:308–315. doi: 10.1016/S0955-2863(98)00022-9. [DOI] [Google Scholar]
- Schweitzer C, Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem Rev. 2003;103:1685–1757. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- Redmond RW, Kochevar IE. Spatially resolved cellular responses to singlet oxygen. Photochem Photobiol. 2006;82:1178–1186. doi: 10.1562/2006-04-14-IR-874. [DOI] [PubMed] [Google Scholar]