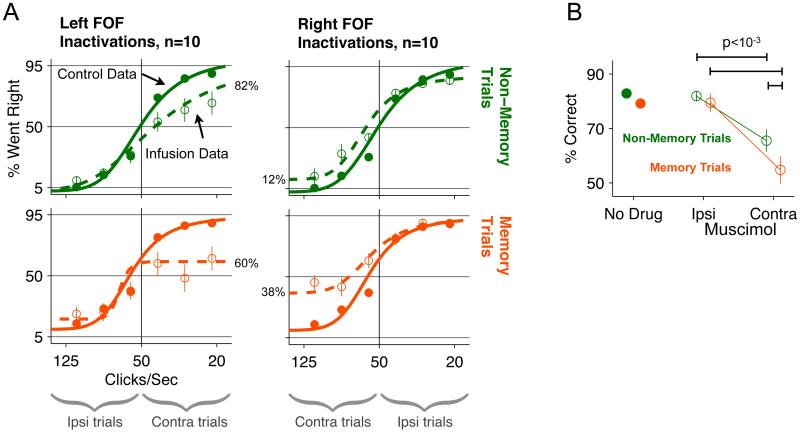
Figure 2. Unilateral inactivation of FOF generates a contralateral impairment that is larger for memory trials compared to non-memory trials.
(A) Behavioral performance on control and muscimol-infusion days. Top row: non-memory trials. Bottom row: memory trials. Left column: muscimol infusions into left FOF. Right column: music-mol infusions into right FOF. Open circles, data from muscimol infusions. Closed circles: control data from days immediately preceding infusion days. Dashed lines: sigmoidal fits to muscimol data. Solid Lines: sigmoidal fits to control data. Error bars are standard error of the mean. Error bars for control data were smaller than the marker in most cases. Underbraces at bottom indicate the sets of trials in which animals were instructed to orient ipsilaterally or contralaterally to the site of infusion. The percentages aligned to the dashed curves indicate the endpoint performance for the trials contralateral to the infusion. (B) Combined data from left and right infusion sessions and collapsed across all stimulus difficulty levels. The “No Drug” data come from the 20 sessions one day before infusion sessions. The Ipsi and Contra Muscimol data are the performance on ipsilateral trials and contralateral trials on infusion sessions (n=20). (See also Figure S2)