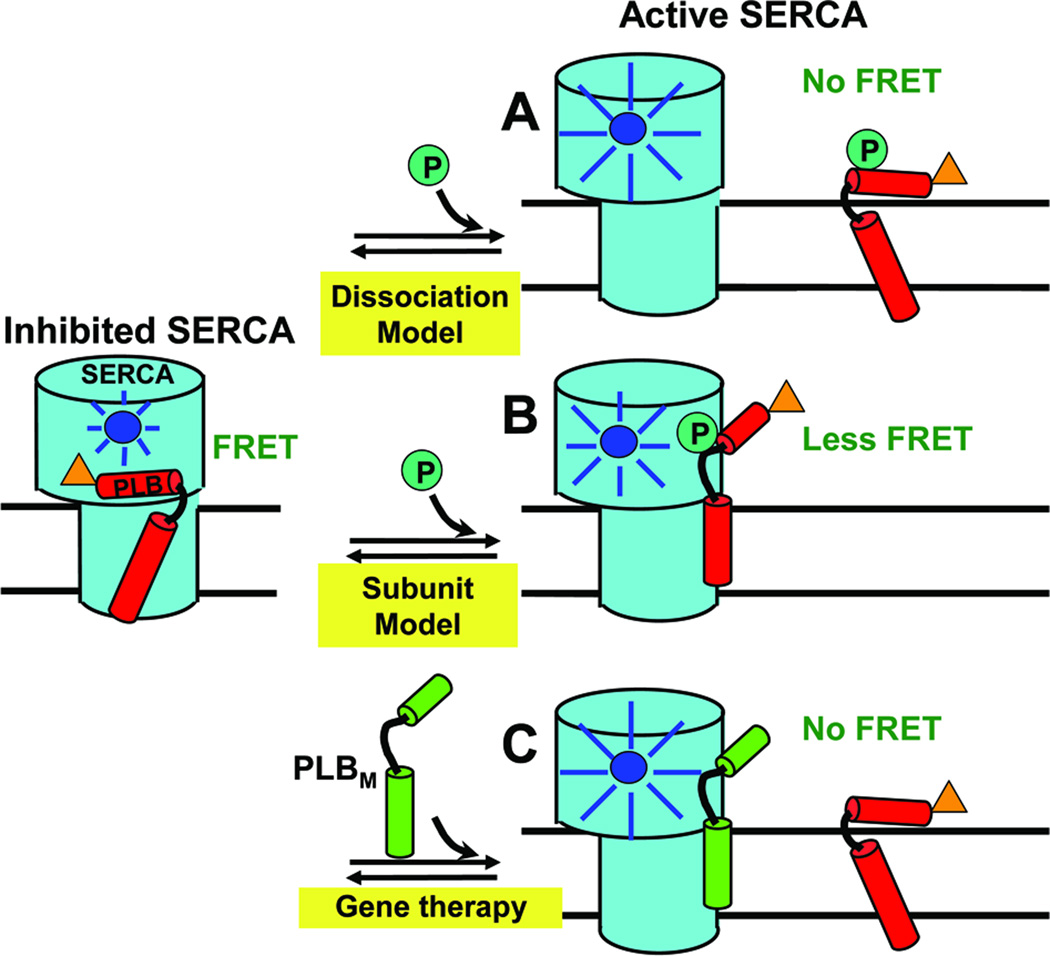
Fig. 1. Models for relief of SERCA inhibition.
Left: PLBW binding to SERCA is detected when the fluorescence of donor (blue) on SERCA is quenched by the acceptor (orange) on PLB via FRET. (A) In the Dissociation Model, loss of function (e.g., due to phosphorylation) requires dissociation of the SERCA-PLB complex, which would completely eliminate FRET. (B) In the Subunit Model, inhibition can be relieved by a structural rearrangement, without dissociation of the SERCA-PLB complex. (C) For HF gene therapy applications, if a LOF mutant PLBM has affinity for SERCA comparable to that of PLBW, it can compete with PLBW to increase SERCA function. These hypotheses can be tested by FRET.