Table 1.
Synthesis of chalcone derivatives by Claisen-Schmidt condensation and their Nrf2 induction activity
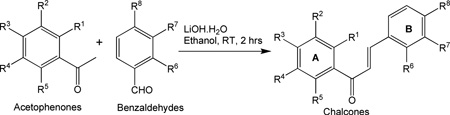 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Chalcones | Substituents on Ring A | Substituents on Ring B |
Relative fold Changea |
|||||||
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | GCLM | NQO1 | ||
| 1 | 1a | H | H | H | H | H | H | H | H | 0.7 | 1.8 |
| 2 | 1b | OMe | H | H | H | H | H | H | H | 3.6 | 3.2 |
| 3 | 1c | H | OMe | H | H | H | H | H | H | 1.5 | 2.4 |
| 4 | 1d | H | H | OMe | H | H | H | H | H | 1.9 | 2.8 |
| 5 | 1e | OMe | H | OMe | H | H | H | H | H | 2.7 | 2.8 |
| 6 | 1f | OMe | H | H | H | OMe | H | H | H | 2.5 | 1.9 |
| 7 | 1g | OMe | H | H | OMe | H | H | H | H | 3.0 | 2.6 |
| 8 | 1h | H | OMe | OMe | H | H | H | H | H | 2.3 | 2.5 |
| 9 | 1i | H | OMe | H | OMe | H | H | H | H | 3.0 | 2.4 |
| 10 | 1j | H | OMe | OMe | OMe | H | H | H | H | 0.7 | 0.7 |
| 11 | 1k | OMe | OMe | OMe | H | H | H | H | H | 2.4 | 2.4 |
| 12 | 1l | OMe | H | OMe | H | OMe | H | H | H | 3.1 | 1.8 |
| 13 | 2a | H | H | H | H | H | CF3 | H | H | 5.0 | 5.3 |
| 14 | 2b | OMe | H | H | H | H | CF3 | H | H | 4.5 | 4.6 |
| 15 | 2c | H | OMe | H | H | H | CF3 | H | H | 5.6 | 4.3 |
| 16 | 2d | H | H | OMe | H | H | CF3 | H | H | 5.4 | 4.6 |
| 17 | 2e | OMe | H | OMe | H | H | CF3 | H | H | 5.4 | 4.5 |
| 18 | 2f | OMe | H | H | H | OMe | CF3 | H | H | 5.7 | 5.3 |
| 19 | 2g | OMe | H | H | OMe | H | CF3 | H | H | 4.4 | 2.7 |
| 20 | 2h | H | OMe | OMe | H | H | CF3 | H | H | 4.3 | 2.7 |
| 21 | 2i | H | OMe | H | OMe | H | CF3 | H | H | 4.0 | 3.7 |
| 22 | 2j | H | OMe | OMe | OMe | H | CF3 | H | H | 3.0 | 3.1 |
| 23 | 2k | OMe | OMe | OMe | H | H | CF3 | H | H | 5.4 | 4.1 |
| 24 | 2l | OMe | H | OMe | H | OMe | CF3 | H | H | 4.4 | 4.3 |
| 25 | 3a | H | H | H | H | H | H | CF3 | H | 3.1 | 2.8 |
| 26 | 3b | OMe | H | H | H | H | H | CF3 | H | 3.9 | 2.9 |
| 27 | 3c | H | OMe | H | H | H | H | CF3 | H | 4.6 | 3.5 |
| 28 | 3d | H | H | OMe | H | H | H | CF3 | H | 2.7 | 2.8 |
| 29 | 3e | OMe | H | OMe | H | H | H | CF3 | H | 4.6 | 4.0 |
| 30 | 3f | OMe | H | H | H | OMe | H | CF3 | H | 1.8 | 0.6 |
| 31 | 3g | OMe | H | H | OMe | H | H | CF3 | H | 1.8 | 0.8 |
| 32 | 3h | H | OMe | OMe | H | H | H | CF3 | H | 4.1 | 2.9 |
| 33 | 3i | H | OMe | H | OMe | H | H | CF3 | H | 3.8 | 3.9 |
| 34 | 3j | H | OMe | OMe | OMe | H | H | CF3 | H | 1.7 | 0.8 |
| 35 | 3k | OMe | OMe | OMe | H | H | H | CF3 | H | 3.6 | 3.2 |
| 36 | 3l | OMe | H | OMe | H | OMe | H | CF3 | H | 1.4 | 0.7 |
| 37 | 4a | H | H | H | H | H | H | H | CF3 | 3.1 | 2.9 |
| 38 | 4b | OMe | H | H | H | H | H | H | CF3 | 4.3 | 5.4 |
| 39 | 4c | H | OMe | H | H | H | H | H | CF3 | 2.9 | 3.1 |
| 40 | 4d | H | H | OMe | H | H | H | H | CF3 | 3.5 | 4.6 |
| 41 | 4e | OMe | H | OMe | H | H | H | H | CF3 | 5.0 | 4.8 |
| 42 | 4f | OMe | H | H | H | OMe | H | H | CF3 | 1.4 | 0.6 |
| 43 | 4g | OMe | H | H | OMe | H | H | H | CF3 | 4.1 | 4.0 |
| 44 | 4h | H | OMe | OMe | H | H | H | H | CF3 | 3.5 | 2.7 |
| 45 | 4i | H | OMe | OMe | OMe | H | H | H | CF3 | 4.1 | 3.7 |
| 46 | 4j | OMe | OMe | OMe | H | H | H | H | CF3 | 4.1 | 3.9 |
| 47 | 4k | OMe | H | OMe | H | OMe | H | H | CF3 | 2.4 | 2.4 |
| 48 | 5a | H | H | H | H | H | NO2 | H | H | 2.1 | 1.6 |
| 49 | 5b | OMe | H | H | H | H | NO2 | H | H | 1.9 | 1.4 |
| 50 | 5c | H | OMe | H | H | NO2 | H | H | 2.9 | 2.7 | |
| 51 | 5d | H | H | OMe | H | H | NO2 | H | H | 4.6 | 4.3 |
| 52 | 5e | OMe | H | OMe | H | H | NO2 | H | H | 2.7 | 3.3 |
| 53 | 5f | OMe | H | H | H | OMe | NO2 | H | H | 4.1 | 3.7 |
| 54 | 5g | OMe | H | H | OMe | H | NO2 | H | H | 1.7 | 0.7 |
| 55 | 5h | H | OMe | OMe | H | H | NO2 | H | H | 2.3 | 2.7 |
| 56 | 5i | H | OMe | H | OMe | H | NO2 | H | H | 3.4 | 4.1 |
| 57 | 5j | H | OMe | OMe | OMe | H | NO2 | H | H | 1.2 | 0.7 |
| 58 | 5k | OMe | OMe | OMe | H | H | NO2 | H | H | 2.9 | 3.3 |
| 59 | 5l | OMe | H | OMe | H | OMe | NO2 | H | H | 2.6 | 2.0 |
| DMSO | 1.0 | 1.0 | |||||||||
| Sulforaphane | 2.7 | 3.6 | |||||||||
Data presented are representative of 3 independent experiments.
Values shown are mean ± SD of quadruplicate wells.
