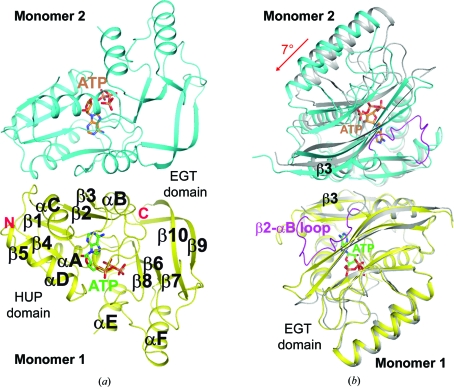
Figure 2.
Crystal structure of the P. furiosus PF0828 homodimer. (a) Schematic drawing of the structure of the PF0828 homodimer in complex with ATP. Monomer 1 is colored yellow and monomer 2 is colored cyan; their bound ATP molecules are shown with green and brown C atoms, respectively. The twofold axis of the dimer is in the horizontal direction. (b) Structural overlay of the PF0828 dimer in complex with ATP (in color) and the free enzyme (gray) viewed down the twofold axis of the dimer. This view is related to that of (a) by a 90° rotation around the vertical axis. Only monomer 1 (in yellow) is included in the overlay and a 7° rotation is observed in the other monomer compared with the apo enzyme. The β2–αB loop is shown in magenta in the ATP complex, while it is mostly disordered in the free enzyme. All structure figures were created with PyMOL (http://www.pymol.org).