Table 1. Crystal parameters, data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Crystal parameters | |
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 83.26, b = 122.06, c = 181.996 |
| Data-collection statistics | |
| Wavelength (Å) | 0.97857 |
| Resolution range (Å) | 34.33–2.4 (2.46–2.4) |
| No. of reflections (measured/unique) | 459689/70340 |
| Completeness (%) | 99.5 (97.6) |
| Rmerge† | 0.127 (0.42) |
| Multiplicity | 6.2 (5.3) |
| Mean I/σ(I) | 13.4 (3.93) |
| Refinement and model statistics | |
| Resolution range (Å) | 34.33–2.4 |
| No. of reflections (work/test) | 70340/3512 |
| Rcryst‡ | 0.151 (0.197) |
| Rfree§ | 0.208 (0.245) |
| R.m.s.d. bonds (Å) | 0.006 |
| R.m.s.d. angles (°) | 0.933 |
| B factors (Å2) | |
| Protein | 34.08 |
| Solvent | 35.92 |
| Phosphate | 33.56 |
| Tris | 42.59 |
| No. of protein atoms | 13163 |
| No. of protein waters | 692 |
| No. of auxiliary molecules | 2 phosphate and 2 Tris |
| Ramachandran plot (%) | |
| Favorable region | 96.1 |
| Additional allowed region | 3.9 |
| PDB entry | 3qde |
R
merge = 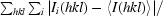
 , where I
i(hkl) is the intensity of an individual measurement of the reflection and 〈I(hkl)〉 is the mean intensity of the reflection.
, where I
i(hkl) is the intensity of an individual measurement of the reflection and 〈I(hkl)〉 is the mean intensity of the reflection.
R
cryst = 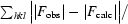
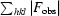 , where F
obs and F
calc are the observed and calculated structure-factor amplitudes.
, where F
obs and F
calc are the observed and calculated structure-factor amplitudes.
R free was calculated as R cryst using a randomly selected 4.9% of the unique reflections that were omitted from the structure refinement.
