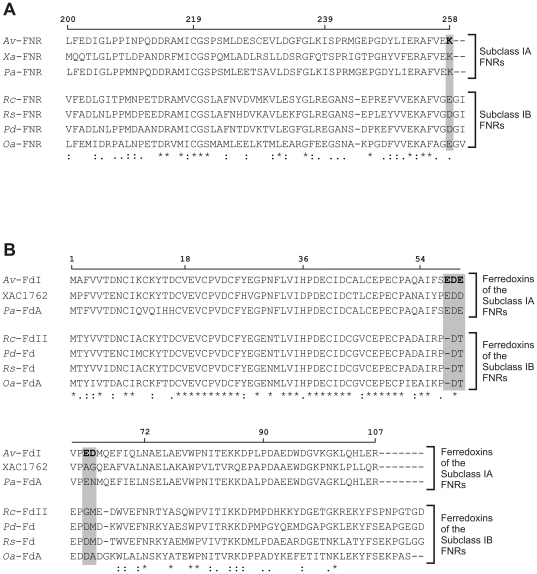
Figure 6. Analysis of Xac-FNR's redox partner by homology sequence.
(A) Alignment of primary structures of subclass I bacterial FNRs from A. vinelandii (Av-FNR, gb: YP_002800963.1), X. axonopodis pv. citri (Xa-FNR, gb: NP_641792.1), Pseudomonas aeruginosa (Pa-FNR, gb: YP_001347117.1), R. capsulatus (Rc-FNR, gb: ADE85336.1), Rhodobacter sphaeroides (Rs-FNR, gb: YP_002524612.1), Paracoccus denitrificans (Pd-FNR, gb: ABL68770.1) and Oceanicaulis alexandrii (Oa-FNR, gb: ZP_00952506.1). Sequence regions from amino acid 200 to the carboxy-terminus are shown. In bold is the amino acid from Av-FNR that is involved in the interaction with ferredoxin I, as was previously reported [42]. (B) Alignment of ferredoxin I from A. vinelandii (Av-FdI, gb: AAA22125.1) and ferredoxins from X. axonopodis pv. citri (XAC1762, gb: NP_642090.1), P. aeruginosa (ferredoxin A, Pa-FdA, gb: AAF89693.1), R. capsulatus (ferredoxin II, Rc-FdII, gb: YP_003578927.1), P. denitrificans (Pd-Fd, gb: ABL69923.1), Rhodobacter sp. (Rs-Fd, gb: ZP_05844833.1) and O. alexandrii (ferredoxin A, Oa-FdA, gb: ZP_00953239.1). In bold is the peptide involved in the interaction with Av-FNR identified by cross-linking experiments, as was previously reported [42]. Potential residues of Av-FdI that interact with Lys258 of Av-FNR are shaded in gray. Numbers over the sequences correspond to the A. vinelandii proteins. The alignments were performed using ClustalX 2.0.11.