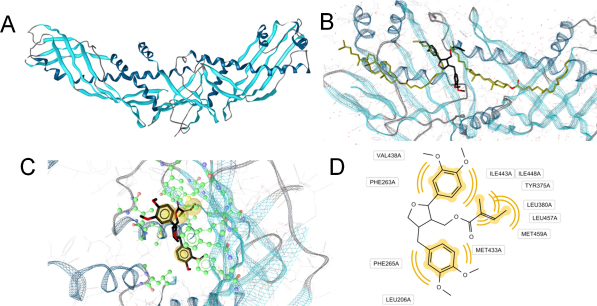
Fig. 4.
Proposed molecular binding mode of leoligin next to the CETP neck region. CETP (A) is a boomerang-shaped protein forming a long, hydrophobic tunnel. In the X-ray crystal structure (B), this tunnel contains two cholesteryl ester molecules (gold). Leoligin (black) was predicted to bind in an area just between these two ligands, next to the CETP neck region. (C) Docking mode of leoligin within the hydrophobic CETP channel. Amino acids involved in direct protein–ligand interactions are colored in green. Substituents of leoligin forming hydrophobic contacts with CETP are highlighted in yellow spheres. (D) 2D representation of leoligin–CETP interactions. Respective interactions are indicated by yellow curves. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)