Abstract
Purpose
The purpose of this study was to elucidate prognostic factors for survival and clinical outcomes of rological soft tissue sarcomas (STSs).
Materials and Methods
This was a retrospective review of the medical records of 48 patients with urological STS treated from January 1982 to July 2009. Demographic and pathological characteristics were compared. Patients' demographics, clinico-pathological parameters, overall survival, and the factors expected to predict survival, such as sex, age at diagnosis, primary organ, surgical resection, metastasis, and mass size, were analyzed. We evaluated differences in survival on the basis of histological subtype by Kaplan-Meier analysis and multivariate Cox proportional hazards regression.
Results
The study included 34 males (70.8%) and 14 females (29.1%). The mean age at diagnosis was 47.1 years (range, 3 to 80). The most common site was the retroperitoneum (n=16), followed by the kidney (n=12), prostate (n=10), bladder (n=7), ureter (n=1), and paratesticular region (n=1). Nineteen patients (39.5%) had other organ metastases at diagnosis. The most common subtypes of sarcoma were leiomyosarcoma (50%), rhabdomyosarcoma (18.7%), and liposarcoma (8%). The remaining 11 cases had other histological subtypes (22.9%). Mean tumor size was 9.5 cm (range, 2.2 to 24). Thirty-three patients (68.7%) underwent surgical resection. The overall survival rate at 5 years was 51.4%. In the univariate and multivariate analysis, surgical resection, primary tumor site, and metastasis at diagnosis remained significant predictors of prognosis. Patients with retroperitoneal sarcoma had a higher overall survival rate by 5 years compared with patients with other organ sarcoma.
Conclusions
The overall survival rate at 5 years was 51.4%. Surgical resection, primary tumor site, and metastasis at diagnosis remained significant predictors of prognosis.
Keywords: Factor, Outcome, Sarcoma, Surgery, Survival
INTRODUCTION
Soft tissue sarcomas (STSs) are a heterogeneous group of solid tumors that arise from embryonic mesenchymal cells and that have distinct clinical and pathological features. Microscopically, arcinosarcomas are biphasic tumors comprising an intimate admixture of carcinomatous and sarcomatous components with an abrupt or gradual transition from one to the other. STSs of the genitourinary (GU) tract are relatively rare, accounting for only 2.1% of all STSs and 1% to 2% of all malignant GU tumors [1-3]. Because of the rarity of urological STSs, clinical data are limited and prognosis is often considered unpredictable [4,5]. Recently, several groups have reported their long-term experiences with GU STSs and have investigated prognosis and prognostic factors [4-6]. Although the consensus is that total surgical resection provides the patient the best chance for cure [2,7], there is no universal agreement concerning prognostic factors for GU STSs. Several analyses of prognostic factors influencing the overall survival (OS) of patients with GU STSs have revealed that tumor stage, grade, size, and anatomic site are important for patient survival.
To the best of our knowledge, no prior study has investigated clinical outcomes and prognostic factors of GU STSs in a Korean patient population. In this study, we investigated prognostic factors of GU STSs on the basis of 28 years of experience with these tumors at a single center in Korea.
MATERIALS AND METHODS
1. Patients
Between January 1982 and July 2009, 48 patients who were histologically diagnosed with a GU STS at our institute were included in this study. Sarcomas of the GU tract that originated from the kidney, ureter, bladder, prostate, paratesticular region, and retroperitoneum were included. Sarcomas that originated from female genital organs such as the uterus, ovary, vulva, or vagina were excluded from this study.
2. Variables
Demographic and clinical data were retrieved by reviewing patients' charts, clinical data, operative notes, radiological reports, and pathological reports. The variables analyzed were patient age, sex, tumor histology, tumor size, primary organ, metastasis at diagnosis, and status of surgical resection. The primary histopathological categories were liposarcoma, leiomyosarcoma, rhabdomyosarcoma, and other STSs. Tumors were divided into two groups on the basis of size: one group comprised tumors smaller than 5 cm, and the other comprised tumors larger than 5 cm. Local recurrence or metastasis was defined as the first recurrence of disease at the primary tumor site or distant site detected by a radiographic modality, such as computed tomography. Morbidity and mortality analyses were conducted by reviewing patient charts and clinical records.
3. Statistical analysis
Univariate analysis of variables to determine prognostic factors was performed by using the log rank test. Multivariate analysis was performed by using the Cox proportional hazards model. The results of the Cox model analysis are reported as hazard ratios and 95% confidence intervals. The Kaplan-Meier estimate of the survival curve was used to summarize the data, whereas differences between patient groups were assessed by the log rank test. Statistical significance was defined as a p<0.05. All statistical tests were performed by using PASW ver. 18.0 software.
RESULTS
1. Patient characteristics
During the study period, 48 patients with GU STSs were treated at our institution. The patients' characteristics are shown in Table 1. There were 14 females (29.1%) and 34 males (70.8%). The mean age at diagnosis was 47.1 years (range, 3 to 80 years). The mean follow-up duration was 43.6 months (range, 6 to 167 months). The most common site was the retroperitoneum (n=16, 33.3%) followed by the kidney (n=12, 25%), prostate (n=10, 20.8%), bladder (n=7, 14.5%), ureter (n=1, 2%), and paratesticular region (n=1, 2%). The mean tumor size was 9.5 cm (range, 2.2 to 24 cm) and 43 patients (89.5%) had tumors larger than 5 cm. Nineteen patients (39.5%) had other organ metastases at diagnosis. The histological subtypes of sarcoma were as follows: leiomyosarcoma (24, 50%), rhabdomyosarcoma (9, 18.7%), and liposarcoma (4, 8%). The remaining 11 cases had other histological subtypes (22.9%). Of the 48 patients, 33 (68.7%) underwent surgical resection and 15 (31.2%) were treated by chemotherapy, radiotherapy, or both.
TABLE 1.
Characteristics of patients with urological soft tissue sarcomas
Values are presented number (%)
2. Survival
At the time of analysis, 20 patients (41.6%) had died of their STS, 2 patients (4.1%) had died of unrelated causes, and 26 patients (54.1%) were alive. The OS rate at 5 years was 51.4% (Fig. 1). The OS rate of inoperable cases at 5 years was 38%. The 5-year estimated survival rate of patients with a retroperitoneal sarcoma was 82%, while that of patients with a bladder sarcoma was 73%. The 5-year estimated survival rate for patients who presented with prostate sarcoma was 44%, while that for patients who presented with renal sarcoma was 39%.
FIG. 1.
Kaplan-Meier analysis of disease specific survival in all patients.
3. Prognostic factors
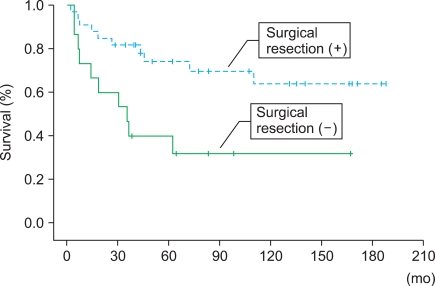
There were no significant differences in survival according to age (p=0.79), gender (p=0.84), tumor size (p=0.59), or histological subtype (p=0.69) according to the univariate analysis (Table 2). However, a significant difference in survival according to metastasis at diagnosis was observed (Fig. 2). Patients who underwent surgical resection also showed significantly better overall survival (Fig. 3). Patients with retroperitoneal sarcomas had more favorable survival outcomes than did patients with bladder, kidney, or prostate sarcomas (Fig. 4). These three factors (metastasis, surgical resection, and primary organ) were also significantly associated with improved overall survival in the multivariate analysis (Table 2).
TABLE 2.
Univariate and multivariate analyses of prognostic factors
CI: confidence interval
FIG. 2.
Kaplan-Meier analysis of overall survival according to presence vs. abscence of metastasis at diagnosis.
FIG. 3.
Kaplan-Meier analysis of overall survival according to surgical resection or not.
FIG. 4.
Kaplan-Meier analysis of overall survival according to primary organ.
DISCUSSION
STSs of the GU tract are rare. Less than 5% of STSs arise in the GU tract and only 15% of STSs arise within the retroperitoneum. The rarity of GU STS is a major obstacle to clinical research; contemporary data in the medical literature are limited. Several earlier clinical studies have investigated urological STSs. Mondaini et al reviewed sarcomas of different histological types in a multicenter study involving eight different hospitals in Tuscany, Italy [5]. The MSKCC group reported two consecutive series, one including 43 patients treated between 1982 and 1989 and another including 131 patients treated between 1977 and 2003 [2]. The latter study was an extension of the former study with a prolonged follow-up. Prognostic factors for local recurrence-free and disease-specific survival were determined [8]. Owing to the rareness of STSs, and therefore the absence of randomized controlled trials, no standard treatment options for this disease exist. Conventional treatment options for STSs include wide margin surgery and radiotherapy. This multimodal approach has replaced amputation as the primary surgical treatment of choice.
In the present study, we analyzed the clinicopathological features of GU STSs and investigated prognostic factors. The mean tumor size of 9.5 cm found in the present study is slightly smaller than that reported previously [9-11]. The 5-year OS was 51.4%, similar to the results of previous studies that reported 5-year OS rates of 50% [10,12].
Several studies have examined predictors of outcome in patients with GU STSs. Dotan et al reported that the presence of metastasis and rhabdomyosarcoma were unfavorable prognostic variables [8]. In our study, the presence of metastasis at diagnosis was associated with survival in both univariate and multivariate analyses. However, no significant difference was detected among the histological subgroups in this study. Lewis et al reported that the presence of unresectable disease and incomplete surgical resection were the most significant factors predictive of disease-specific death [13]. In a study by van Dalen et al of 143 patients treated in the Netherlands, complete tumor resection was correlated with better overall survival in the multivariate analysis [14]. However, Dotan et al reported that complete resection was not a significant factor predictive of disease-specific survival in univariate and multivariate analysis in 102 patients with primary tumors only [15]. These results suggest that any type of surgical resection can provide the best chance of survival in patients presenting with primary disease or with primary and metastatic disease. Consistent with this, we found that the absence of surgical resection was an unfavorable prognostic variable for overall survival. Therefore, we suggest that surgical resection may contribute to a favorable prognosis in patients with urological STSs.
The prognosis of retroperitoneal STSs has been reported to be more favorable than that of the other GU STSs. Disease prognosis was the worst for STSs of the kidney, whereas the prognosis was better for retroperitoneum, bladder, and prostate sarcomas, as reported previously [7].
According to a previous study, the size of STSs is an important prognostic variable [16], in contrast with our findings. This discrepancy may be because STSs arising from the retroperitoneum can attain a large size owing to the flexibility of the retroperitoneum. However, retroperitoneal STSs showed a better prognosis than bladder, prostate, and renal STSs in our study.
The histological subtype of the GU STSs was not a prognostic factor for disease-specific survival in either the univariate or the multivariate analysis; in other words, we did not observe an association between histological subtype and disease-specific survival. However, the small number of patients in each group made statistical comparisons difficult.
CONCLUSIONS
Genitourinary sarcomas are a rare group of tumors with a generally poor prognosis. The overall survival rate at 5 years was 51.4%. Surgical resection, metastasis at diagnosis, and the primary organ were significant predictors of prognosis. Retroperitoneal STSs had more favorable outcomes than bladder, prostate, and renal STSs. The data presented in this study contribute to our understanding of urological STSs and may help in the development of an optimal therapeutic strategy to treat STSs.
Footnotes
The authors have nothing to disclose.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Russo P, Brady MS, Conlon K, Hajdu SI, Fair WR, Herr HW, et al. Adult urological sarcoma. J Urol. 1992;147:1032–1036. doi: 10.1016/s0022-5347(17)37456-6. [DOI] [PubMed] [Google Scholar]
- 3.Stojadinovic A, Leung DH, Allen P, Lewis JJ, Jaques DP, Brennan MF. Primary adult soft tissue sarcoma: time-dependent influence of prognostic variables. J Clin Oncol. 2002;20:4344–4352. doi: 10.1200/JCO.2002.07.154. [DOI] [PubMed] [Google Scholar]
- 4.Izumi K, Mizokami A, Sugimoto K, Narimoto K, Miyagi T, Maeda Y, et al. Role of surgical resection in adult urological soft tissue sarcoma: 25-year experience. Urol Int. 2010;84:309–314. doi: 10.1159/000288234. [DOI] [PubMed] [Google Scholar]
- 5.Mondaini N, Palli D, Saieva C, Nesi G, Franchi A, Ponchietti R, et al. Clinical characteristics and overall survival in genitourinary sarcomas treated with curative intent: a multicenter study. Eur Urol. 2005;47:468–473. doi: 10.1016/j.eururo.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura J, Morii E, Takahashi T, Souma Y, Nakajima K, Doki Y, et al. Abdominal soft tissue sarcoma: a multicenter retrospective study. Int J Clin Oncol. 2010;15:399–405. doi: 10.1007/s10147-010-0075-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G, Chen KK, Manivel C, Fraley EE. Sarcomas of the retroperitoneum and genitourinary tract. J Urol. 1989;141:1107–1110. doi: 10.1016/s0022-5347(17)41184-0. [DOI] [PubMed] [Google Scholar]
- 8.Dotan ZA, Tal R, Golijanin D, Snyder ME, Antonescu C, Brennan MF, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176:2033–2038. doi: 10.1016/j.juro.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez JC, Perez EA, Franceschi D, Moffat FL, Jr, Livingstone AS, Koniaris LG. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res. 2007;141:105–114. doi: 10.1016/j.jss.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Pacelli F, Tortorelli AP, Rosa F, Papa V, Bossola M, Sanchez AM, et al. Retroperitoneal soft tissue sarcoma: prognostic factors and therapeutic approaches. Tumori. 2008;94:497–504. doi: 10.1177/030089160809400410. [DOI] [PubMed] [Google Scholar]
- 12.Perez EA, Gutierrez JC, Moffat FL, Jr, Franceschi D, Livingstone AS, Spector SA, et al. Retroperitoneal and truncal sarcomas: prognosis depends upon type not location. Ann Surg Oncol. 2007;14:1114–1122. doi: 10.1245/s10434-006-9255-x. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dalen T, Plooij JM, van Coevorden F, van Geel AN, Hoekstra HJ, Albus-Lutter Ch, et al. Long-term prognosis of primary retroperitoneal soft tissue sarcoma. Eur J Surg Oncol. 2007;33:234–238. doi: 10.1016/j.ejso.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Dotan ZA, Tal R, Golijanin D, Snyder ME, Antonescu C, Brennan MF, et al. Adult genitourinary sarcoma: The 25-year memorial sloan-kettering experience. J Urol. 2006;176:2033–2039. doi: 10.1016/j.juro.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan RC, A'Hern R, Fisher C, Thomas JM. Modified staging system for extremity soft tissue sarcomas. Ann Surg Oncol. 1999;6:57–69. doi: 10.1007/s10434-999-0057-9. [DOI] [PubMed] [Google Scholar]