Abstract
Fragile X syndrome is the most common cause of inherited intellectual impairment and the most common single-gene cause of autism. Individuals with fragile X syndrome present with a neurobehavioural phenotype that includes selective deficits in spatiotemporal visual perception associated with neural processing in frontal–parietal networks of the brain. The goal of the current study was to examine whether reduced resolution of spatial and/or temporal visual attention may underlie perceptual deficits related to fragile X syndrome. Eye tracking was used to psychophysically measure the limits of spatial and temporal attention in infants with fragile X syndrome and age-matched neurotypically developing infants. Results from these experiments revealed that infants with fragile X syndrome experience drastically reduced resolution of temporal attention in a genetic dose-sensitive manner, but have a spatial resolution of attention that is not impaired. Coarse temporal attention could have significant knock-on effects for the development of perceptual, cognitive and motor abilities in individuals with the disorder.
Keywords: crowding, flicker, magnocellular, Mooney, contrast sensitivity
Introduction
Fragile X syndrome is the most prominent form of inherited intellectual impairment, affecting ∼1 in 3600 males and 1 in 4000 females, and is also the most common monogenetic cause of autism (Crawford, 2001; Beckett et al., 2005; Fernandez-Carvajal et al., 2009). Fragile X syndrome results from an expansion mutation of 200 or more CGG trinucleotide repeats in the promoter region of the fragile X mental retardation 1 (FMR1) gene on the X chromosome (Verkerk et al., 1991). The mutation is associated with methylation and transcriptional silencing of the gene and consequently leads to a reduced level or complete loss of fragile X mental retardation protein (FMRP; Devys et al., 1993). Insufficient FMRP gives rise to abnormal dendritic spine maturation akin to that found in animals deprived of sensory experience, and to deficient synaptic pruning during brain development (Greenough et al., 2001; Bagni and Greenough, 2005). Since the genetic and molecular aetiology of fragile X syndrome is now well-defined, it offers a unique opportunity to examine the functional role of a single gene product in neurocognitive development. The primary phenotype of fragile X syndrome includes mild to severe intellectual impairment; however, this impairment is not general in nature. Rather, individuals with fragile X syndrome present with a specific profile of strengths and weaknesses characterized by an imbalance both within and between domains. Affected individuals demonstrate relative strength in performance on tasks implicating vocabulary, memory and visual matching, but weakness in performance on tasks of inhibitory control, numerical processing, selective and sustained attention, visual–spatial integration and motor coordination (Schneider et al., 2009). This particular profile of performance in individuals with fragile X syndrome has generally been interpreted as reflecting developmental deficits across a widely distributed network of functional cortical areas including the parietal and frontal lobes. Whether or not a single abnormality in an underlying neural mechanism lies at the root of these functionally related impairments is unclear. Here, we focus on examining whether there is a specific deficit in the resolution of spatial and/or temporal visual attention that may play a significant, if not primary, contributing role in the reported developmental differences found in fragile X syndrome.
A growing body of evidence has revealed that individuals with fragile X syndrome exhibit selective impairments in spatiotemporal visual processing; that is, the processing of information that changes location over time. Studies using behavioural psychophysics in adult males with fragile X syndrome have reported reduced contrast sensitivity for visual stimuli presented at high temporal frequencies known to engage the magnocellular, but not parvocellular, pathway (Kogan et al., 2004). The magnocellular pathway begins with retinal ganglion cells that project to the magnocellular layers of the lateral geniculate nucleus and ends in primary visual cortex (Merigan and Maunsell, 1993). Kogan et al. (2004) used immunohistochemistry to confirm that neurons in the magnocellular layers of the lateral geniculate nucleus from an autopsied brain of an adult male with fragile X syndrome were abnormally small and displayed no FMRP staining, indicating an anatomical susceptibility to absence of the protein. Reduced perceptual sensitivity for both static and moving visual stimuli defined by differences in texture, accompanied by near normal sensitivity for static stimuli defined by differences in luminance, has also been found in adult males with fragile X syndrome (Kogan et al., 2004). These authors take these results to suggest that individuals with fragile X syndrome possess a deficit in transmission of dynamic visual information between subcortical and higher level cortical visual areas.
Impairments in processing of spatiotemporal visual information have recently been discovered in infants and toddlers with fragile X syndrome. Infants with fragile X syndrome show significantly reduced sensitivity to the detection of texture-defined dynamic stimuli, although they are capable of detecting static patterns and luminance-defined dynamic patterns at a level comparable with developmental age-matched infants (Farzin et al., 2008). Further evidence for a selective deficit in processing dynamic information comes from the finding that infants with fragile X syndrome exhibit an inability to maintain a mental representation of, and track, an occluded object during a spatiotemporal transformation, but can represent the occluded object when it remains static (Farzin and Rivera, 2010). There is an intimate link between the processing of spatial and temporal information, such that precise temporal discrimination of discrete events in space is fundamental for determining ‘what’ went ‘where’, as is needed for motion perception (Cavanagh, 1992; Culham et al., 1998). Critically, detection of texture-defined motion and tracking of objects are both mediated by visual attention (Seiffert and Cavanagh, 1998; Scholl, 2001), and involve processing that is independent from low-level, or automatic luminance-based motion processing that occurs even in the absence of attention to the stimulus (Derrington et al., 2004).
Abnormalities in visual attention have also been found in toddlers with fragile X syndrome. Using touch-screen technology, visual search performance of these toddlers has been characterized as having more repetitive errors to previously found targets, and more incorrect touches to distractors, particularly when distractors were similar to targets and when more than one distractor was present (Scerif et al., 2004, 2007). The results highlight a deficit in selective visual attention in toddlers with fragile X syndrome, and are consistent with the well-documented deficits in inhibitory control found in older children and adults with the disorder.
The goal of the present study was to examine the precise nature of the spatiotemporal visual processing deficits previously found in individuals with fragile X syndrome by disentangling spatial from temporal attention limits in infants diagnosed with the disorder compared with attention limits obtained from developmental age-matched neurotypical infants. We hypothesized that both spatial and temporal visual attention would independently show coarser resolutions (reduced limits) in infants with fragile X syndrome relative to neurotypical infants. This prediction was based on the characteristic cognitive profile observed in individuals with fragile X syndrome and on the critical role that FMRP is known to play in the development of brain structure and connectivity. Psychophysical measures of these visual abilities would indicate whether, for example, atypical motion perception or object tracking is a secondary consequence of elementary impairments in spatial and/or temporal processing or a deficit specific to motion per se. Furthermore, we investigated whether individual differences in spatial and temporal visual attention are directly related to molecular measures of the expansion mutation of the gene.
Experiment 1: crowding as a measure of spatial resolution of attention
Extensive psychophysical studies have established that adult spatial vision is fundamentally limited by crowding—the reduced ability to identify an object in the periphery when it is surrounded by other objects (Whitney and Levi, 2011). This limit extends beyond that of visual acuity, because even though the object is still visible, it is not readily identifiable.
Crowding is the operational measure of spatial resolution of attention. When multiple objects fall within a peripheral region of the visual field, features within and between the objects appear jumbled, impairing identification of the target object (He et al., 1996; Cavanagh et al., 1999; Intriligator and Cavanagh, 2001). Crowding has been demonstrated using gratings, numbers, letters and faces. In the periphery, spatial resolution decreases with increasing eccentricity and with reduced target-flanker spacing. Crowding is thought to be minimal or non-existent at the fovea (Bouma, 1970, 1973; Bouma and Andriessen, 1970; Andriessen and Bouma, 1976; Tripathy and Cavanagh, 2002; Pelli et al., 2004; Louie et al., 2007; Levi, 2008; Pelli and Tillman, 2008).
Here, we utilized our recently developed eye-tracking paradigm (Farzin et al., 2010) to measure the extent of spatial crowding in infants with fragile X syndrome compared with developmental age-matched neurotypical infants.
Method
Participants
Participants included in this experiment were 32 infants diagnosed with the fragile X syndrome full mutation (13 females, mean chronological age = 26.10 ± 13.15 months, range = 6–46 months). For this and the following experiments, infants with fragile X syndrome were recruited from and clinically evaluated at the MIND Institute Fragile X Research and Treatment Centre as part of a larger longitudinal study on visual and cognitive development of infants with fragile X syndrome. Infants with the disorder underwent a routine blood test to confirm the presence of the full mutation (>200 CGG repeats). Genomic DNA was isolated from peripheral blood lymphocytes using standard methods (Puregene Kit; Gentra Inc.), and analysis and calculation of the CGG repeat size were carried out using an Alpha Innotech FluorChem 8800 Image Detection System (Tassone et al., 2000). FMR1 messenger RNA level was quantified using a 7900 Sequence detector (PE Biosystems) as previously described (Tassone et al., 2000). Since larger expansions of CGG repeats (>200) typically result in reduced levels or absence of FMR1 messenger RNA and the gene's protein product, FMRP, we examined the relationship between these molecular measures and performance on the psychophysical tasks to gain further insight into the role of FMRP in brain development.
The developmental level of infants with fragile X syndrome was evaluated by a trained researcher using the Mullen Scales of Early Learning standardized assessment (MSEL; Mullen, 1995), and revealed a mean developmental age of 17.23 months [standard deviation (SD) = ± 10.24 months, range = 4–32 months]. Infants with fragile X syndrome were matched based on their developmental age to a group of 32 neurotypical infants, who were chronologically younger in age than the participants with fragile X syndrome (10 females, mean chronological age = 15.28 ± 5.69 months, range = 6–22 months). There was no significant difference in developmental level between the neurotypical controls and the infants with fragile X syndrome, based on the assumption that neurotypical infants’ developmental age was approximately equivalent to their chronological age [F(1, 63) = 2.78, P = 0.101]. Neurotypical infants were recruited through letters to families, fliers and word of mouth in Davis, California and surrounding areas. All participants had uncorrected visual acuity. The study was approved by the Institutional Review Board at the University of California, Davis, and informed consent was obtained from a parent or legal guardian of all infants.
Apparatus and stimuli
The experiment has been described in detail previously (Farzin et al., 2010). The experiment was programmed in Presentation version 11.3 (Neurobehavioral Systems, Inc.) and stimuli were presented on a Tobii 17-inch LCD binocular eye tracker monitor.
Stimuli consisted of 10 Mooney faces, five of which were used in the original Mooney study (Mooney, 1957). Mooney faces do not have facial features that can be identified using bottom-up or image-based processes; rather, top-down, holistic processing is required to perceive the image first as a face and only then identify facial features (Cavanagh, 1991; Moore and Cavanagh, 1998; Kemelmacher-Shlizerman et al., 2008).
In adults, upright Mooney faces activate known face-selective brain areas such as the fusiform face area (Andrews and Schluppeck, 2004; George et al., 2005; Latinus and Taylor, 2005) and are more easily and rapidly identified as faces when they are in an upright rather than inverted orientation. Recent developmental work has shown that infants prefer to look at upright rather than inverted Mooney faces (Doi et al., 2009; Leo and Simion, 2009; Farzin et al., 2010).
Faces were 99.77% Michelson contrast, and were cropped to fit into a 5° by 3° ellipse when viewed from a distance of 60 cm. Six 1.53° by 1.05° flankers were created by ‘cutting’ elliptically shaped sections from each upright target face. In the crowded condition, flankers were presented surrounding the target faces at a fixed horizontal centre-to-centre distance of 2.2° between the target face and the flanker. Stimuli were presented against a grey background (77.23 cd/m2; Fig. 1).
Figure 1.
(A) Mooney face stimuli used in Experiment 1; (B) upright face without flankers; (C) inverted face without flankers; (D) upright face with flankers; and (E) inverted face with flankers.
Procedure
All experiments began with a five-point calibration routine on the eye tracker to estimate the infant's gaze position accurately during the task. Trials began with a 1° central fixation video until the infant's gaze was obtained within a 1° radius around the video. Trials in which an infants’ central fixation was not obtained within 10 s, or which shifted outside of the radius before the faces were presented [on average four trials per infant, no group difference [t(62) = 1.52, P = 0.134], were discarded. Immediately following the central fixation video, one upright and one inverted Mooney face (the same identity) were shown, one face to the left and one to the right of fixation at a centre-to-centre distance of either 3, 6 or 10° along the horizontal meridian. Both faces were presented with (crowded) or without (uncrowded) the six corresponding flanker parts for 2 s (Fig. 1). The eccentricity at which the faces were presented was blocked in sets of four trials, and the order of blocks, side of upright face and the presence of flankers were randomized.
Data coding and threshold estimation
Eye-tracking data were coded offline using Noldus Observer 5.0 software, and statistical analyses were performed with SPSS (version 16.0). The primary measure of performance on each trial was the location of infants’ first fixation from the central video immediately following the onset of the faces. A fixation was defined as a series of data points within a 30 pixel radius for a minimum duration of 100 ms. A first fixation was coded as a hit (1) if it was on the upright face and a miss (0) if it was on the inverted face. In order to make a first saccade that landed on the upright face, the infant must have perceived the upright face in the periphery. If the infant's gaze remained at the centre of the screen or the infant's first fixation was on an area of the screen that was not on a face, a score of 0.5 was given, based on the assumption that these fixation behaviours did not indicate discrimination between the stimuli. Correct performance was thereby calculated as proportion of first fixations made to the upright face.
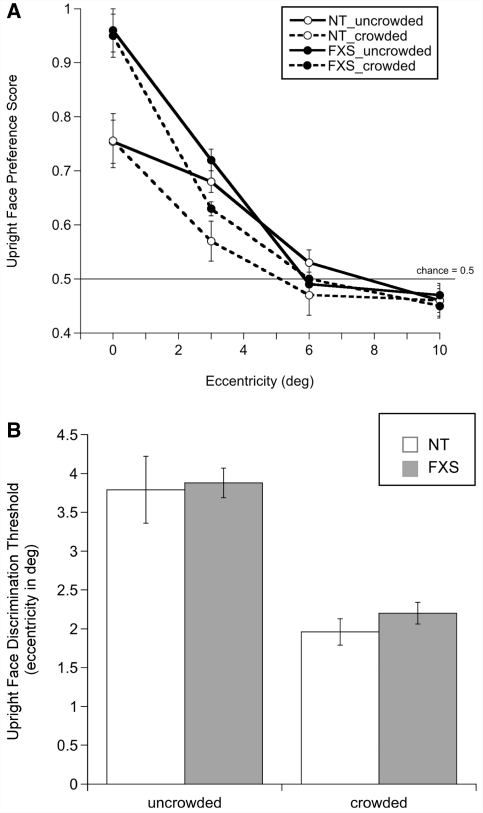
A diagnostic criterion for crowding is that it rarely occurs in the fovea (Pelli et al., 2004). To confirm the lack of a crowding effect during foveal viewing, we separately calculated an upright face preference score, indexing the proportion of time the infant spent fixating the upright face. Preferential looking to the upright face was computed for each trial by dividing the time spent fixating the upright face by the total time spent fixating both faces, ranging from 0 (never looked at upright face) to 1 (only looked at upright face), with 0.5 considered the chance level. Upright face preference scores were equivalent at the three eccentricities so the average score was used as a measure of foveal (0°), or free-viewing, performance on each trial. Because the infant’s gaze was directed to the upright face, the image must therefore have been projected onto the fovea. This measure allowed us to verify both that infants with fragile X syndrome do exhibit a face inversion effect akin to neurotypical infants and that foveal performance did not differ between uncrowded and crowded face conditions for each group of infants.
A logistic function was fit to each infant's data as a function of eccentricity and crowding condition using the psignifit toolbox for MATLAB (version 2.5.6), which implements the maximum-likelihood procedure described by Wichmann and Hill (2001). For all participants, the upper asymptote of the function was fixed at 1 (ceiling performance) and the lower limit was set to 0.5 (chance performance). To estimate parameters, threshold, slope and error, a bootstrapping technique was used which included 5000 replications for each fitted function. The criterion for including infants in the analyses was the goodness of fit of the function, evaluated using deviance scores >2 SDs above the mean of the group of infants (Wichmann and Hill, 2001). Two infants with fragile X syndrome were excluded from the analyses. Threshold was defined as the eccentricity value yielding upright face discrimination performance of 0.75. Higher threshold values indicate greater spatial resolution of attention.
Results
There was no difference in the number of trials successfully completed by infants in each group [neurotypical: mean = 50.34, SD = 19.57; fragile X syndrome: mean = 44.00, SD = 13.23; F(1, 63) = 2.31, P = 0.134]. Additionally, there was no difference in performance between male and female infants within each group, so this variable was removed from further analyses.
A repeated measures mixed-model ANOVA with eccentricity (0, 3, 6, 10°) and crowding (uncrowded or crowded faces) as within-subject factors and group (neurotypical or fragile X syndrome) as a between-subject factor was conducted on performance. Unless otherwise noted, all P-values reported are Bonferroni corrected for multiple comparisons. A significant main effect of eccentricity [F(3, 60) = 89.95, P = 0.0001, ηp2 = 0.818] and significant interaction effects between eccentricity and crowding [F(3, 60) = 4.53, P = 0.006, ηp2 = 0.185] and eccentricity and group [F(3, 60) = 3.16, P = 0.031, ηp2 = 0.137] were identified. The same ANOVA with the foveal (0°) eccentricity condition removed verified the presence of a significant main effect of eccentricity [F(2, 61) = 82.89, P = 0.0001, ηp2 = 0.731] and a significant interaction effect between eccentricity and crowding [F(2, 61) = 6.294, P = 0.003, ηp2 = 0.171], confirming that the crowding effect did not differ with or without the upright face preference score. These results revealed that infants’ ability to discriminate the upright Mooney face in the periphery decreased as a function of eccentricity, and was significantly worse when the faces were crowded (Fig. 2A). The interaction effect between eccentricity and group was driven by higher foveal performance in infants with fragile X syndrome compared with neurotypical infants in both the uncrowded and crowded conditions. Contrary to our prediction, no significant group difference was found in either analysis, indicating that crowding did not differ between infants with and without fragile X syndrome.
Figure 2.
(A) Mean upright face preference score (±SEM) as a function of eccentricity for uncrowded and crowded faces, by group; (B) Mean upright face discrimination threshold (±SEM) for uncrowded and crowded faces, by group. Higher thresholds signify higher resolution of spatial attention. FXS = fragile X syndrome; NT = neurotypical.
To further examine the effect of flankers on upright face preference for each group of infants, we conducted within-group paired-samples t-tests (two-tailed) at each eccentricity. Both groups of infants showed a significant difference between performance in the uncrowded and the crowded conditions when faces were presented at 3° [neurotypical: t(31) = 2.14, P = 0.040; fragile X syndrome: t(31) = 4.31, P = 0.0001], but not at further eccentric locations. At 6 and 10°, infants’ performance was not different from change in either the uncrowded or crowded conditions. Critically, flankers did not impair visual preference for the upright face when viewed foveally, consistent with the definition of crowding and distinguishing it from a masking process, which would have prevented both detection and discrimination independent of eccentricity.
Individual infant upright face discrimination thresholds from psychometric function fits were analysed using a repeated measures mixed-model ANOVA with crowding (uncrowded or crowded faces) as the within-subject factor and group (neurotypical or fragile X syndrome) as the between-subject factor, which yielded a significant main effect of crowding [F (1, 62) = 72.55, P = 0.0001, ηp2 = 0.539], reflecting higher eccentricity thresholds (better performance) when the faces were uncrowded. No other effects were found. Pair-wise t-tests (two-tailed) confirmed significantly higher sensitivity in the uncrowded (neurotypical: mean = 3.79, SD = 2.48; fragile X syndrome: mean = 3.89, SD = 1.09) compared with the crowded (neurotypical: mean = 1.97, SD = 0.97; fragile X syndrome: mean = 2.24, SD = 0.79) condition in both groups of infants [neurotypical: t(31) = 5.25, P = 0.0001; fragile X syndrome: t(31) = 7.73, P = 0.0001]. Therefore, flankers impacted upright face discrimination equally in infants with and without fragile X syndrome (Fig. 2B).
To compare the effect of crowding between individual infants, a threshold difference score was calculated by subtracting the threshold obtained for crowded faces from the threshold obtained for uncrowded faces. Difference scores were significantly positive for both groups [neurotypical: t(31) = 2.17, P = 0.038; fragile X syndrome: t(31) = 4.31, P = 0.0001], reflecting higher eccentricity limits for uncrowded faces. This analysis verifies that crowding-specific processes reduced upright face discrimination performance and that the decrement in discrimination did not differ between groups.
We also examined the relationship between uncrowded and crowded thresholds and other variables of interest. For neurotypical controls, chronological age was positively correlated with uncrowded threshold values [r(32) = 0.384, P = 0.030] such that older infants exhibited greater eccentricity limits for discrimination of the upright face presented in isolation. For infants with fragile X syndrome, there was no significant relationship between age and either threshold measure. Additionally, no significant relationship was found between molecular variables (CGG repeat length and FMR1 messenger RNA levels) and spatial thresholds in infants with fragile X syndrome.
Discussion
The goal of this experiment was to use visual crowding to quantify the spatial resolution of visual attention in infants with fragile X syndrome compared with neurotypical infants. The psychophysical measure of upright face discrimination thresholds established that infants with fragile X syndrome were able to identify the uncrowded Mooney face to a limited extent in the periphery and that crowding interfered with identification at 3°. Thresholds for uncrowded and crowded face discrimination in infants with fragile X syndrome were equivalent to those measured in developmental age-matched neurotypical infants, suggesting that spatial resolution is intact in infants with fragile X syndrome.
The use of Mooney faces as stimuli had the advantage of allowing us to examine several aspects of face processing abilities in infants with fragile X syndrome. This is the first study to examine face detection in infants with fragile X syndrome. We found that infants with fragile X syndrome were able to perceive Mooney faces, as illustrated by a selective visual preference for the upright relative to the inverted face. Since Mooney faces lack individual facial features and cannot be recognized by bottom-up processes, a preference for the upright face suggests that, at the ages tested, individuals with fragile X syndrome possess an intact holistic face processing system. Holistic face processing is thought to rely on analysis of the configuration or relations between features, and therefore demonstrates Gestalt processing of the face as a whole unit (Tanaka and Farah, 1993). Further, these results, for the first time, establish the presence of the face inversion effect (Yin, 1969) in individuals with fragile X syndrome. Lastly, given that infants with fragile X syndrome showed no gaze aversion to the faces, these data suggest that avoidance of social stimuli may develop after infancy, perhaps as a coping mechanism in response to increased social demands.
Crowding is believed to impose the fundamental bottleneck preventing object-level visual information from reaching conscious awareness (Levi, 2008; Pelli and Tillman, 2008; Whitney and Levi, 2011), thereby providing a quantitative measure of the resolution of spatial attention (Intriligator and Cavanagh, 2001) and allowing us to study the development of spatial attention in infants with fragile X syndrome. The current findings revealed no difference in the resolution of spatial attention between infants with and without fragile X syndrome. One explanation for our findings is that spatial attention in isolation may be relatively unaffected in infants with fragile X syndrome, but that a deficit lies in the integration of spatial and temporal attention. Previous reports of visual processing impairments in individuals with fragile X syndrome utilized tasks that did not separate these two types of attention, and therefore required simultaneous attention to both static and dynamic information. The crowding task used in this experiment aimed to identify the limit of spatial attention for the recognition of static faces in the peripheral visual field, eliminating temporal attention. It is also plausible that the spatial resolution of attention required for visual perception is different from the spatial resolution required for visually guided actions, such as pointing or grasping (Bulakowski et al., 2009), and that the latter is selectively impacted in individuals with fragile X syndrome. Accordingly, identification of a target in a visual search task would be more impacted by the clutter, or crowding, caused by distractors, as was found in toddlers with fragile X syndrome (Scerif et al., 2004). These two explanations need not be mutually exclusive, as spatial and temporal information must be combined for accurate visuomotor responses.
Although chronological age predicted uncrowded thresholds in neurotypical controls, it did not do so for infants with fragile X syndrome. This may be the result of a measurable increase in peripheral visual acuity, particularly for medium to high spatial frequencies, in the younger aged neurotypical infants, but less so in the older age range of the infants with fragile X syndrome. This reasoning is in line with evidence that processing of fine detail is dependent on a sensitive window of postnatal visual experience (Maurer and Lewis, 1993; Dobkins et al., 1999, 2009; Birch and O'Connor, 2001).
Infants in both groups spent a greater proportion of time fixating the upright face, whether uncrowded or crowded. The finding that infants with fragile X syndrome spent an even greater proportion of time fixating the upright face relative to neurotypical controls was unexpected and may be accounted for by delayed disengagement from the upright face. At eccentricities beyond the fovea, performance in both the uncrowded and crowded conditions eventually dropped to chance levels, which is expected for peripheral face recognition (Mäkelä et al., 1993, 2001; McKone, 2004). This decline in performance is likely the result of both reduced acuity and within-face crowding in the periphery (Martelli et al., 2005; Farzin et al., 2009). Nevertheless, because infants were able to direct their first fixation precisely to either the upright or inverted face as far as 10° eccentricity, we are certain that infants were able to ‘perceive’ the stimuli in their periphery and it is therefore unlikely that acuity limits alone explain the decline in uncrowded performance.
At this point, the resolution of spatial visual attention in older children, adolescents and adults with fragile X syndrome remains unknown. Hence, although the limits of resolution are comparable between infants with and without fragile X syndrome early in development, we do not know if attentional resolution reaches an asymptotic level in individuals with fragile X syndrome that is lower compared with that of developmental age-matched neurotypical individuals. Literature on the maturation of spatial visual attention in neurotypical individuals is also scarce so it is yet to be determined at what age adult levels of resolution are attained. In any case, the spatial resolution of infant attention is comparable in those with and without fragile X syndrome.
Experiment 2: phase individuation as a measure of temporal resolution of attention
Similar to spatial attention, temporal attention also has a limited resolution (Battelli et al., 2003, 2007, 2008). The temporal interval over which the visual system is able to segregate information is referred to as temporal resolution. The human adult visual system has been found to have two broad classes of temporal limits; a higher limit for perception of flicker or simple motion and a lower limit for identification of rapidly changing events (Battelli et al., 2007, 2008; Holcombe, 2009). For example, though adults can detect the presence of flickering light at rates of up to 60 Hz, they cannot readily individuate between the light and dark states of the flicker beyond 7–10 Hz (Verstraten et al., 2000; Battelli et al., 2001, 2003; Aghdaee and Cavanagh, 2007). Temporal individuation is the operational measure of temporal resolution of attention.
Experiment 1 found that infants with fragile X syndrome had an equivalently coarse spatial resolution of visual attention as that of developmental age-matched neurotypical infants. It therefore remains possible that reduced resolution of temporal attention alone may underlie spatiotemporal visual impairments observed in individuals with fragile X syndrome. To determine the temporal resolution of attention in infants with fragile X syndrome relative to developmental age-matched neurotypical infants, we psychophysically measured infants’ temporal individuation thresholds using a modified version of the phase discrimination task used previously with adults (Aghdaee and Cavanagh, 2007; Battelli et al., 2007), and which we have recently used with neurotypical infants and adults (Farzin et al., 2011). The task is ideal because it isolates specifically the temporal aspect of event individuation.
Method
Participants
Twenty-one infants diagnosed with the fragile X syndrome full mutation (seven females, mean chronological age = 24.20 ± 8.17 months, range = 6–37 months) were included in this experiment; 15 of whom were also included in Experiment 1. Infants with fragile X syndrome had a mean developmental age of 15.17 months (SD = ± 8.04 months, range = 2–26 months). The infants were matched based on their developmental age to a group of 21 neurotypical infants (five females, mean chronological age = 14.21 ± 2.33 months, range = 6–16 months). There was no significant difference in developmental level between the neurotypical infants and infants with fragile X syndrome [t(40) = −0.793, P = 0.432].
Apparatus
The apparatus was identical to that of Experiment 1.
Procedure
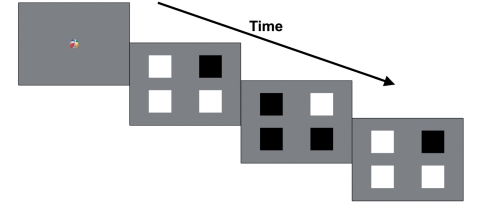
Following calibration of the eye tracker, trials began with a 1° fixation video presented at the centre of the screen for 1 s. The task was a four-alternative forced-choice method of constant stimuli preferential looking paradigm. After the fixation video (0 ms delay), four squares subtending 2.5 × 2.5° of visual angle were presented centred 5° to the left and right of fixation against a grey background (77.24 cd/m2). The target square was chosen randomly from one of the four square locations. All squares underwent square-wave flicker between white (133.8 cd/m2) and black (0.26 cd/m2) states, but the target was 180° out-of-phase from the three distractors (Fig. 3). For example, the target was always black when the three distractors were white, and vice versa. Flickering occurred at one of four temporal frequencies: 0.2, 0.5, 1 or 2 Hz. At slower rates, the target is more easily seen because it is easier to individuate the black and white states, but at frequencies faster than the threshold level all squares appear to be flickering identically (i.e. the phase cannot be individuated). Therefore, if infants can individuate the phase of the squares, they should show a visual preference for the target square. Trial duration was for 5 s, and eight trials were presented at each temporal frequency, in random order.
Figure 3.
Schematic illustration of three frames from Experiment 2.
Data coding and threshold estimation
Infants from whom data were recorded on at least half of the trials were included in the final analysis (six infants with fragile X syndrome and two neurotypical infants were excluded). Coding was similar to that described in Experiment 1 except for as noted below. Fixation position was coded by dividing the screen into four quadrants. A target preference score, indexing the proportion of looking time to the target square, was calculated for each trial by dividing the time spent looking at the target square by the total time spent looking at all four squares. Target preference scores ranged from 0 (never looked at target) to 1 (only looked at target), with 0.25 considered the chance level. For each infant, an average target preference score was calculated at each temporal frequency.
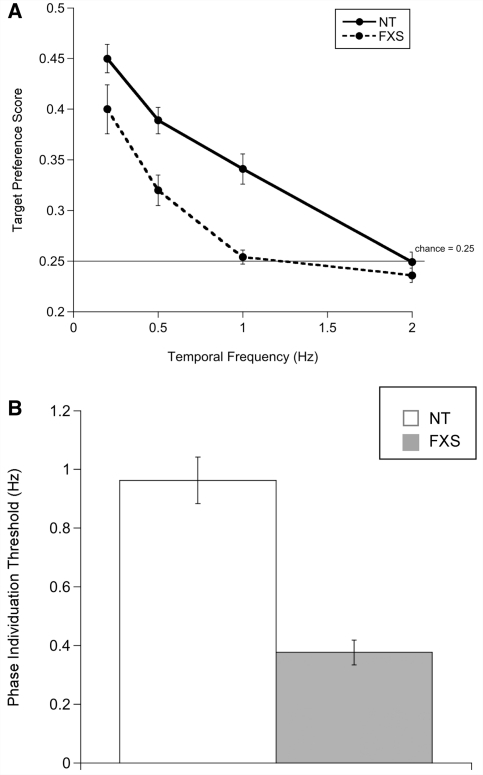
To obtain each infant's phase individuation threshold, a logistic function was fit to the target preference scores as a function of temporal frequency. Because the peak target preference score across both groups of infants was 0.65 at the slowest temporal frequency (0.2 Hz), the upper asymptote (ceiling) of the function was fixed at 0.70, in order to improve the fit to the data (Dobkins et al., 1999). The lower limit was set to 0.25 (chance performance). Phase individuation threshold level was thus defined as the temporal frequency yielding half the asymptotic performance, corresponding to a target preference score of 0.475, which is equivalent to using the 75% performance correct threshold level for a typical two-alternative forced-choice task. Higher temporal frequency thresholds indicate greater temporal resolution of attention.
Results
There were no significant sex differences within group for target preference score or for temporal discrimination threshold. Average target preference score as a function of temporal frequency for infants in each group is shown in Fig. 4A. A repeated measures mixed-model ANOVA with temporal frequency (0.2, 0.5, 1, 2 Hz) as the within-subject factor and group (neurotypical or fragile X syndrome) as the between-subject factor revealed a significant main effect of temporal frequency [F(3, 38) = 60.21, P = 0.0001, ηp2 = 0.826], whereby both groups of infants had significantly higher target preference scores at lower temporal frequencies. In addition, an interaction was found between temporal frequency and group [F(3, 38) = 5.28, P = 0.004, ηp2 = 0.294], reflecting significantly better phase discrimination ability in neurotypical infants relative to infants with fragile X syndrome. Two-tailed independent samples t-tests were conducted to qualify group differences in target preference at each temporal frequency. The results confirmed that neurotypical infants exhibited a significantly greater visual preference for the target square compared with infants with fragile X syndrome at 0.5 Hz [t(40) = 3.55, P = 0.001], 1 Hz [t(40) = 5.10, P = 0.0001] and 2 Hz [t(40) = 2.01, P = 0.052]. Note, however, that neither neurotypical infants nor infants with fragile X syndrome differed from the chance preference score of 0.25 at 2 Hz [neurotypical: t(20) = −0.090, P = 0.459; fragile X syndrome: t(20) = −0.027, P = 0.929]. The target preference scores reveal that neurotypical infants were able to identify the out-of-phase flicker up to a rate of 1 Hz, whereas infants with fragile X syndrome could do so only up to 0.5 Hz.
Figure 4.
(A) Mean target preference score (±SEM) as a function of temporal frequency, by group; (B) mean phase individuation threshold by group (±SEM). Higher thresholds signify higher resolution of temporal attention. FXS = fragile X syndrome; NT = neurotypical.
There was no overall difference in mean looking times between groups across temporal frequency levels [F(3, 38) = 0.186, P = 0.905, ηp2 = 0.014], ruling out the possibility that lower target preference scores were due to shorter looking times or general perceptual or attentional differences in infants with fragile X syndrome.
A phase individuation threshold was calculated for each infant, and a one-way ANOVA was carried out to compare thresholds between groups. A significant effect of group was found [F(1, 41) = 42.59, P = 0.0001, ηp2 = 0.516], driven by higher temporal sensitivity in neurotypical infants (mean = 0.96, SD = 0.36) than in infants with fragile X syndrome (mean = 0.38, SD = 0.19), as illustrated in Fig. 4B.
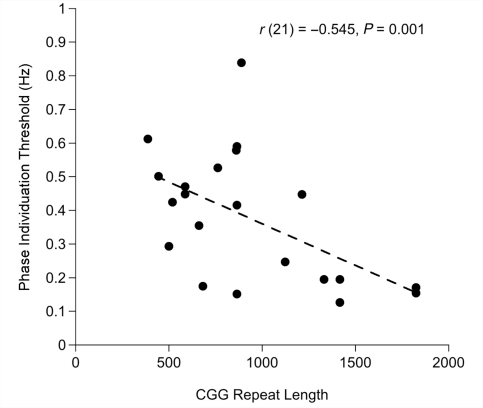
Pearson's correlation analysis was used to evaluate the relationship between molecular variables (CGG repeat length and FMR1 messsenger RNA level) and phase individuation thresholds in infants with fragile X syndrome. These analyses established a significant negative correlation between CGG repeat length and threshold [r(21) = −0.545, P = 0.011], confirmed using the bootstrap resampling and permutation methods (P < 0.01; Efron and Tibshirani, 1993). This link revealed that infants with longer CGG repeat lengths had more reduced temporal sensitivity (Fig. 5). No significant relationship was found between chronological or developmental age and threshold in the group of neurotypical infants. However, the correlation between developmental age and threshold trended towards a significant positive correlation in infants with fragile X syndrome [r(21) = 0.416, P = 0.061], signifying greater temporal sensitivity in developmentally older infants.
Figure 5.
Individual infant phase individuation threshold plotted against CGG repeat length for infants with fragile X syndrome.
Discussion
Experiment 2 quantified the resolution of temporal attention in infants with fragile X syndrome, revealing dramatically lower rates of phase discrimination compared with developmental age-matched neurotypical infants. These findings demonstrate a selective deficit in the resolution of temporal visual attention in infants with fragile X syndrome, and critically, the extent of the perceptual deficit was found to be related in a dose-sensitive fashion to CGG repeat length in the FMR1 gene.
Abnormally coarse temporal resolution of conscious visual perception likely impacts the development of other perceptual, cognitive and motor skills, particularly those functions that require accurate and precise temporal sensitivity. Given the dynamic nature of the visual environment, the ability to perceive the identity of rapidly changing events is vital for nearly all activities. For an infant, these activities may range from planning and executing the timing of eye movements in order to engage in a social interaction to predicting when to reach out your hands to catch a ball. While the full extent of the relationship between temporal visual attention and atypical cognitive development in individuals with fragile X syndrome requires further investigation, our results indicate that reduced temporal attention may serve as a window into the visual processing impairments that are hallmarks of the disorder.
Reduced or absent FMRP production is the exclusive genetic cause of fragile X syndrome, so understanding the biological genotype is essential for uncovering the phenotypic outcome. While some studies have shown that the pattern of overall cognitive deficit in individuals with fragile X syndrome can be predicted from the number of CGG repeats or the level of FMRP expression (Loesch et al., 1993; Abrams et al., 1994; Rousseau et al., 1994), others have not found a simple direct mapping between molecular status and phenotype (de Vries et al., 1993; Reiss et al., 1995; Cornish et al., 2001). The widely accepted explanation for this inconsistency has been that individuals with higher numbers of CGG repeats possess a version of the gene that is fully methylated and thus produces no FMRP. Therefore, complete absence of protein expression amounts to less phenotypic variability in individuals with the full mutation (particularly males). Existing measures of FMRP have been imprecise, preventing exact measurement of protein levels across the spectrum of CGG repeat lengths. With the advent of new quantitative tools, such as the enzyme-linked immunosorbent assay method (Iwahashi et al., 2009), a wider range of protein expression has been found across the spectrum of CGG repeat lengths, particularly in the lower end of the full mutation range of repeats. Our understanding of the direct relationship between FMRP level and the various fragile X syndrome-associated clinical phenotypes is likely to improve in the near future.
The decrease in resolution of temporal visual attention in infants with fragile X syndrome is consistent with prior work describing a selective deficit in the detection of texture-defined dynamic stimuli in infants with fragile X syndrome (Farzin et al., 2008), which, thus far, appears to be specific to infants with fragile X syndrome (Bertone et al., 2010). Texture-defined motion perception is mediated by attention and critically depends on the temporal discrimination of events in space (Cavanagh and Mather, 1989; Cavanagh, 1992; Seiffert and Cavanagh, 1998). Therefore, the temporal processing deficit observed here likely originates from the same abnormality in neural processing underlying texture-defined motion detection in individuals with fragile X syndrome. This interpretation is also consistent with the numerous reports of visual attention deficits in individuals with fragile X syndrome when measured using tasks that require temporal discrimination (Cornish et al., 1998, 1999, 2001; Munir et al., 2000; Scerif et al., 2004, 2005, 2007).
Individuation of dynamic visual events, in this case light and dark phases of flicker, requires not only temporal attention but also the ability to detect rapid luminance changes that are generated during contrast reversals. It is therefore possible that the deficit found in Experiment 2 is due to inability of the infants with fragile X syndrome to perceive the flickering squares; a basic loss in the temporal resolution of vision rather than attention per se. At high temporal frequencies, our ability to perceive that a change has occurred is nearly an order of magnitude greater than our ability to register the identity of what changed, and it is known that these processes are carried out at different levels of the visual system. Detection of rapid luminance changes occurs in the magnocellular pathway, which, if dysfunctional, may lead to impaired temporal vision. Abnormal lower level visual processing could then have implications on neural networks across the developing brain, including higher level areas involved in temporal attention. This hypothesis is tested in Experiment 3.
Experiment 3: flicker detection as a measure of temporal resolution of vision
The magnocellular pathway is characterized mainly by large cells with fast conducting axons that are sensitive to high temporal frequencies and to low to medium spatial frequencies. Thus, cells in the magnocellular pathway respond to rapid changes in illumination, such as flicker (Kaplan and Shapley, 1986). The parvocellular pathway, on the other hand, is characterized by smaller cells with more slowly conducting axons that are sensitive to high spatial frequencies and colour, and manifests a lower sensitivity to temporal frequencies. It is therefore important to establish whether the purported subcortical processing difference in adults with fragile X syndrome is present in infants with fragile X syndrome and whether underlying neuroanatomical and functional abnormalities specific to the magnocellular pathway may impact temporal attention in this developmental population.
Experiment 3 psychophysically measured contrast sensitivity at two spatial and temporal frequencies, chosen to preferentially favour a magnocellular (low spatial frequency/high temporal frequency) or parvocellular (high spatial frequency/low temporal frequency) pathway response. If infants with fragile X syndrome show reduced contrast sensitivity for high temporal frequency flicker, it would support the theory that magnocellular pathway cells rely more strongly on FMRP than parvocellular pathway cells, and would provide a low-level explanation for the reduced resolution of temporal attention found in Experiment 2. Alternatively, if infants with fragile X syndrome demonstrate intact ability to detect contrast at higher temporal frequencies than the limits found in Experiment 2, it would discount a subcortical temporal processing explanation and suggest that higher level temporal attention must be responsible.
Method
Participants
Twenty-seven infants diagnosed with the fragile X syndrome full mutation (nine females, mean chronological age = 25.11 ± 14.04 months, range = 6–42 months; mean developmental age = 16.07 ± 10.14, range = 4–30 months) were included in this experiment; 16 of whom were also included in Experiment 2. Twelve infants with fragile X syndrome participated in all three experiments. Six additional infants with fragile X syndrome were tested, but failed to provide sufficient gaze data for threshold estimation. A group of 26 developmental age-matched neurotypical infants (10 females, mean chronological age = 15.14 months, SD = 2.15, range = 6–16 months) were included for comparison purposes. There was no significant difference in developmental level between the neurotypical controls and the infants with fragile X syndrome [t(51) = −1.104, P = 0.275].
Apparatus
The apparatus was identical to that of Experiments 1 and 2. Stimuli were generated using The Vision Shell PPC program, controlled by an Apple G4 Power Macintosh.
Procedure
Infants’ contrast sensitivity was obtained using a two-alternative forced-choice method of constant stimuli preferential looking paradigm, as previously described by Farzin et al. (2008). Stimuli consisted of vertically oriented Gabors with a single luminance-defined sinusoid. Spatial frequency was either 0.2 cycles/degree (low spatial frequency) with the phase of each Gabor reversing sinusoidally at 10 Hz (high temporal frequency) or 5 cycles/degree (high spatial frequency) reversed at 2 Hz (low temporal frequency; Fig. 6A). These parameters were chosen to match the response properties of magnocellular and parvocellular cells, respectively. Michelson contrast was defined as the difference between the maximum and minimum luminance of the gratings, divided by their sum, and four contrast levels were presented (14, 19, 28, 42%) with 10 trials per level, in random order. The stimulus appeared on either the left or right half of the screen, counterbalanced for side and was presented within a 3-s Gaussian window, fading in and out of view. The non-stimulus half of the screen was equiluminant grey. In between each trial, a 1° fixation video was presented to reorient infants’ fixation to the centre of the screen. To keep the duration of testing manageable for infants, the stimulus conditions (magnocellular or parvocellular) were presented separately and in random order. However, a number of infants with fragile X syndrome (n = 14) and neurotypical control infants (n = 8) were particularly compliant and provided sufficient gaze data to estimate contrast sensitivity for both conditions.
Figure 6.
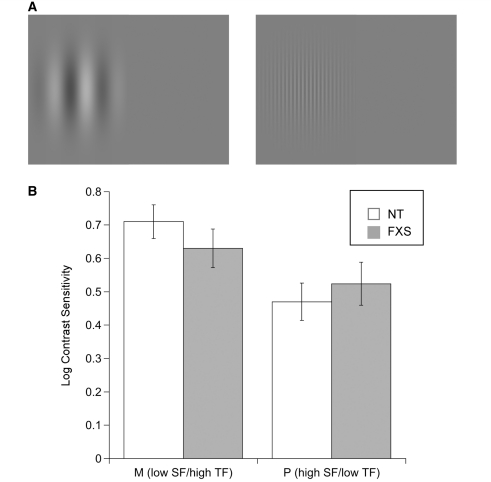
(A) Schematic illustration of the stimuli used in Experiment 3. Spatial frequency was 0.2 cycles/degree (low spatial frequency) with the phase of each Gabor reversing sinusoidally at 10 Hz (high temporal frequency; left), or was 5 cycles/degree (high spatial frequency) reversing at 2 Hz (low temporal frequency; right). (B) Mean log contrast sensitivity for magnocellular (M; low spatial frequency/high temporal frequency) and parvocellular [P; high spatial frequency/low temporal frequency stimuli (±SEM)], by group. FXS = fragile X syndrome; NT = neurotypical; SF = spatial frequency; TF = temporal frequency.
Data coding and threshold estimation
Fixation position on the screen was coded as left, right, centre or away. A visual preference score, indexing the proportion of looking time to the stimulus side of the screen, was calculated by dividing the amount of time spent looking at the side of the screen with the stimulus by the total time spent looking at both sides. Visual preference scores ranged from 0 to 1, with 0.5 considered performance at the chance level. Trials in which no fixations occurred were considered missing trials and were not given a preference score or included in the final calculation. For each infant, a mean visual preference score was calculated at each contrast level.
Contrast sensitivity was calculated by fitting a logistic function to individual infants’ average visual preference scores as a function of contrast using the psignifit toolbox for MATLAB. Threshold performance was defined as the contrast yielding a score of 0.75, and sensitivity was computed as the inverse of the threshold. Sensitivity values were then logged to conform to normal distributions (Graham, 1989).
Results
Twenty-two infants with fragile X syndrome completed the low spatial frequency/high temporal frequency stimulus condition and 19 completed the high spatial frequency/low temporal frequency condition. Twenty-four neurotypical infants completed the low spatial frequency/high temporal frequency stimulus condition and 10 completed the high spatial frequency/low temporal frequency condition. Figure 6B shows mean log contrast sensitivities for each stimulus condition (low spatial frequency/high temporal frequency or high spatial frequency/low temporal frequency) as a function of group. For each stimulus condition, a one-way ANOVA was performed to compare mean log contrast sensitivities as a function of group. The result yielded no significant group difference in sensitivity for low spatial frequency/high temporal frequency stimuli [F(1, 45) = 1.203, P = 0.279, ηp2 = 0.027] or for high spatial frequency/low temporal frequency stimuli [F(1, 29) = 0.359, P = 0.554, ηp2 = 0.013]. Consistent with earlier studies, neurotypical infants who completed both flicker detection conditions showed significantly higher sensitivity for detecting low spatial frequency/high temporal frequency stimuli (mean = 0.59, SD = 0.281) relative to their sensitivity for detecting high spatial frequency/low temporal frequency stimuli (mean = 0.51, SD = 0.166) [t(7) = 6.096, P = 0.0001]. Infants with fragile X syndrome who completed both conditions also showed higher sensitivity for low spatial frequency/high temporal frequency stimuli (mean = 0.61, SD = 0.31) relative to high spatial frequency/low temporal frequency stimuli (mean = 0.49, SD = 0.203); however, the difference did not reach a significance level of 0.05.
As in the other two experiments, we performed correlation analyses between each of the molecular measures (CGG repeat length and FMR1 messenger RNA level) and contrast sensitivity within infants in the fragile X syndrome group and found no significant relationships. Also, there was no significant association between chronological or developmental age and contrast sensitivity for either stimulus condition in either group of infants. Contrast sensitivities for the two stimulus conditions were correlated with each other in neurotypical infants [r(8) = 0.792, P = 0.019].
Discussion
The purpose of Experiment 3 was to measure contrast sensitivity for low spatial frequency/high temporal frequency (magnocellular stimuli) and high spatial frequency/low temporal frequency (parvocellular stimuli) luminance gratings in infants with fragile X syndrome to determine whether a basic deficit in the perception of temporal visual information may account for the reduced temporal attention limit found in Experiment 2. The results here indicate that infants with fragile X syndrome were able to detect flicker at temporal frequencies of 2 and 10 Hz at contrast levels equivalent to that of developmental age-matched neurotypical infants. The Michelson contrast of the flickering stimuli used in Experiment 2 was nearly 100%, and thereby well above infants’ detection thresholds. We therefore attribute the results of Experiment 2 to an inability to individuate the phase of the flicker—a coarser resolution of temporal attention—rather than to limited low-level temporal contrast sensitivity.
These findings are not supportive of a magnocellular pathway deficit as the source of visual processing impairments previously reported in individuals with fragile X syndrome. While work by Kogan et al. (2004) found reduced contrast sensitivity for low spatial frequency gratings temporally modulated at high temporal frequency in a small group of adolescent and adult males with fragile X syndrome using a yes–no staircase detection task, here we find no difference in contrast sensitivity between infants with and without fragile X syndrome. It is difficult to explain with certainty the source of the difference in the findings. One interpretation is that the role of FMRP in the early development of these subcortical visual areas may follow a protracted time course and our results may be uncovering one end of an atypical developmental trajectory, prior to the emergence of a magnocellular pathway deficit. Measures of contrast sensitivity during the childhood years, and ideally, longitudinally, beginning in infancy, should be obtained in future studies to fully understand the developmental trajectory of visual processing in fragile X syndrome. Alternatively, because there is some overlap in the spatial and temporal ranges of the magnocellular and parvocellular pathways (Derrington and Lennie, 1982), it is possible that the parameters of the stimuli used did not uniquely tap the magnocellular system and therefore elicited parvocellular responses in both stimulus conditions [while Kogan et al. (2004) used 18 Hz, we used 10 Hz to more closely match the contrast sensitivity functions of young infants]. That the contrast sensitivity values obtained from infants with fragile X syndrome in both stimuli conditions is nearly indistinguishable from those of neurotypical infants, and that they follow the expected pattern of higher magnocellular than parvocellular sensitivity (Dobkins et al., 1999; Atkinson, 2000; Hammarrenger et al., 2003) suggest that the observed effects may not entirely be explained by differences in stimulus parameters. Further, these results are in-line with previous data from infants with fragile X syndrome revealing a selective deficit in sensitivity for detecting texture-defined, but not luminance-defined, dynamic stimuli (Farzin et al., 2008).
General discussion
The ability to spatially and temporally select and attend to information that is relevant to our current behavioural goals is a fundamental aspect of everyday vision. This skill is particularly important during infancy when vision is the primary sensory modality with which infants inspect and learn from their environment. In adults, there are well-established limits of spatial and temporal visual resolution, beyond which, information does not reach conscious awareness. The overall aim of this study was to measure the resolution of spatial and temporal attention in infants with fragile X syndrome, a developmental disorder of known single-gene origin and which has been associated with selective impairments in spatiotemporal visual processing. Results from Experiment 1 established that infants with fragile X syndrome experience a spatial resolution of visual perception equivalent to that of developmental age-matched neurotypical infants; infants in both groups were able to discriminate an upright Mooney face in the presence of surrounding flankers up to a limit of ∼3° in the periphery. In contrast, infants with fragile X syndrome experience significantly poorer temporal resolution relative to developmental age-matched neurotypical infants; phase individuation limits in those with fragile X syndrome were half the temporal frequency at which neurotypical infants could individuate events. Overall, these results reveal that there is a selective temporal attention deficit in infants with fragile X syndrome that is directly related to the molecular genotype of the individual infant. Reduced temporal attention may therefore underlie many of the visual deficits previously reported in individuals with the disorder, including impairments in motion perception and visual–motor coordination (Cornish et al., 1999; Munir et al., 2000; Kogan et al., 2004a, b; Baranek et al., 2005; Mazzocco et al., 2006; Farzin et al., 2008).
The ‘when’ pathway of the brain is dedicated to representing temporal information in order to determine when objects appear or disappear, or to keep track of object displacements and transformations. This pathway is thought to originate in primary visual cortex and involve visual motion area MT+, with the right posterior parietal lobe and prefrontal cortex serving as the core anatomical loci (Battelli et al., 2007, 2008). It is possible that parietal networks are functionally impaired from infancy in individuals with fragile X syndrome, either as a result of disrupted synaptic pruning or FMRP-deficiency-related axonal pathology. This interpretation is consistent with findings of early abnormal neurodevelopment in the absence of FMRP (Hoeft et al., 2010).
Research has established that FMRP is involved in typical neuronal maturation, including synaptic formation and axon development (Abitbol et al., 1993; Comery et al., 1997; Jin and Warren, 2000; Irwin et al., 2002; Antar et al., 2004). It has also been suggested that reduced levels of FMRP causes a disruption in the pruning of dendritic spines, resulting in immature, elongated spines morphologically similar to those found in neurons of animals deprived of sensory experience (Greenough et al., 2001). Furthermore, in patients with fragile X syndrome, the density of these immature spines is elevated when compared with normal brains, suggesting a lack of appropriate synaptic pruning (Irwin, 2000). Such dendritic abnormalities have been found in occipito-parietal and visual cortices of autopsied tissue from patients with fragile X syndrome and Fmr1 knock-out mice (Irwin et al., 2002), suggesting that FMRP is an important protein in the development of visual areas of the brain. These structural abnormalities are likely to impact functions that rely on integration of information across areas, as is the case for networks supporting temporal processing. The regions involved in the ‘when’ pathway may be particularly impacted as a result of abnormal dendritic spine morphology characteristic of the fragile X syndrome disorder.
Conclusion
The current series of experiments are the first to investigate spatial and temporal visual attention in infants with fragile X syndrome, designed around the hypothesis that spatiotemporal visual processing abnormalities characteristic of individuals with fragile X syndrome may arise from coarser spatial and/or temporal visual resolution. Here, we report drastically reduced resolution of temporal, but not spatial, visual attention that was directly linked to the extent of the genetic trinucleotide repeat mutation in the FMR1 gene. Temporal visual processing plays a direct role in many other perceptual, cognitive and motor abilities, including motion perception and visual–motor coordination, and may hold promise as a syndrome-specific early diagnostic marker or treatment outcome measure for fragile X syndrome and other developmental disorders.
Funding
National Institutes of Health (MH083386 to F.F., HD056031 to S.M.R. and EY018216 to D.W.); National Science Foundation (0748689 to D.W.).
Acknowledgements
We are grateful to Dr Flora Tassone for providing molecular data, Jason Fischer for programming Experiment 3 and Kristi Hendrickson for assistance in testing participants.
Glossary
Abbreviations
- FMR1
fragile X mental retardation 1
- FMRP
fragile X mental retardation protein
References
- Abitbol M, Menini C, Delezoide AL, Rhyner T, Vekemans M, Mallet J. Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nat Genet. 1993;4:147–53. doi: 10.1038/ng0693-147. [DOI] [PubMed] [Google Scholar]
- Abrams MT, Reiss AL, Freund LS, Baumgardner TL, Chase GA, Denckla MB. Molecular-neurobehavioral associations in females with the fragile X full mutation. Am J Hum Genetics. 1994;51:317–27. doi: 10.1002/ajmg.1320510407. [DOI] [PubMed] [Google Scholar]
- Aghdaee SM, Cavanagh P. Temporal limits of long-range phase discrimination across the visual field. Vis Res. 2007;47:2156–63. doi: 10.1016/j.visres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Schluppeck D. Neural responses to Mooney images reveal a modular representation of faces in human visual cortex. NeuroImage. 2004;21:91–8. doi: 10.1016/j.neuroimage.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Andriessen JJ, Bouma H. Eccentric vision: adverse interactions between line segments. Vis Res. 1976;16:71–8. doi: 10.1016/0042-6989(76)90078-x. [DOI] [PubMed] [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile X mental retardation protein and Fmr1 mRNA localization differentially in dendrites and synapses. J Neurosci. 2004;24:2648–55. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. The developing visual brain. Oxford: Oxford Medical Publication; 2000. [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: The roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–87. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Danko CD, Skinner ML, Bailey DBJ, Hatton DD, Roberts JE, et al. Video analysis of sensory-motor features in infants with fragile X syndrome at 9-12 months of age. J Autism Dev Disord. 2005;35:645–56. doi: 10.1007/s10803-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Henaff MA, Michel F, et al. Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron. 2001;32:985–95. doi: 10.1016/s0896-6273(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Martini P, Barton JJ. Bilateral deficits of transient visual attention in right parietal patients. Brain. 2003;12:2164–74. doi: 10.1093/brain/awg221. [DOI] [PubMed] [Google Scholar]
- Battelli L, Pascual-Leone A, Cavanagh P. The ‘when' pathway of the right parietal lobe. Trends Cognit Sci. 2007;11:204–10. doi: 10.1016/j.tics.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli L, Walsh V, Pascual-Leone A, Cavanagh P. The ‘when' parietal pathway explored by lesion studies. Curr Opin Neurobiol. 2008;18:120–6. doi: 10.1016/j.conb.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett L, Qilu Y, Long AN. The impact of fragile X: prevalence, numbers affected, and economic impact. A white paper prepared for the National Fragile X Foundation. National Fragile X Foundation Quarterly Journal. Davis, CA; 2005. http://www.fragilex.org/pdf/PrevalenceWhitePaperAdaptedforFQ.pdf (18 October 2011, date last accessed)
- Bertone A, Hanck J, Kogan CS, Chaudhuri A, Cornish K. Using perceptual signatures to define and dissociate condition-specific neural etiology: autism and fragile X syndrome as model conditions. J Autism Dev Disord. 2010;40:1531–40. doi: 10.1007/s10803-010-1109-5. [DOI] [PubMed] [Google Scholar]
- Birch EE, O'Connor AR. Preterm birth and visual development. Semin Neonatol. 2001;6:487–97. doi: 10.1053/siny.2001.0077. [DOI] [PubMed] [Google Scholar]
- Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226:177–8. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- Bouma H. Visual interference in the parafoveal recognition of initial and final letters of words. Vis Res. 1973;13:767–82. doi: 10.1016/0042-6989(73)90041-2. [DOI] [PubMed] [Google Scholar]
- Bouma H, Andriessen JJ. Induced changes in the perceived orientation of line segments. Vis Res. 1970;4:333–49. doi: 10.1016/0042-6989(70)90104-5. [DOI] [PubMed] [Google Scholar]
- Bulakowski PF, Post RB, Whitney D. Visuomotor crowding: the resolution of grasping in cluttered scenes. Front Behav Neurosci. 2009;3:1–7. doi: 10.3389/neuro.08.049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P. Attention-based motion perception. Science. 1992;257:1563–5. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. What's up in top-down processing? In: Gorea A, editor. Representations of vision: trends and tacit assumptions in vision research. Cambridge: Cambridge University Press; 1991. p. 295–304. [Google Scholar]
- Cavanagh P, He S, Intriligator J. Attentional resolution: the grain and locus of visual awareness. In: Taddei-Ferretti C, Musio C, editors. Neuronal basis and psychological aspects of consciousness. Singapore: World Scientific; 1999. pp. 41–52. [Google Scholar]
- Cavanagh P, Mather G. Motion: the long and short of it. Spat Vis. 1989;42:103–29. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Differential impact of the FMR-1 full mutation on memory and attention functioning: a neuropsychological perspective. J Cognit Neurosci. 2001;13:144–50. doi: 10.1162/089892901564126. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Spatial cognition in males with fragile-X syndrome: evidence for a neuropsychological phenotype. Cortex. 1999;35:263–71. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. The nature of the spatial deficit in young females with Fragile-X syndrome: a neuropsychological and molecular perspective. Neuropsychologia. 1998;36:1239–46. doi: 10.1016/s0028-3932(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Crawford D, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review [Review] Genet Med. 2001;3:359–71. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell B. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–70. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- de Vries BB, Wiegers AM, de Graaff E, Verkerk AJ, Van Hemel JO, Halley DJ, et al. Mental status and fragile X expression in relation to FMR-1 gene mutation. Eur J Hum Genetics. 1993;1:72–9. doi: 10.1159/000472389. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Allen HA, Delicato LS. Visual mechanisms of motion analysis and motion perception. Ann Rev Psychol. 2004;55:181–205. doi: 10.1146/annurev.psych.55.090902.141903. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. The influence of temporal frequency and adaptation level on receptive-field organization of retinal ganglion-cells in cat. J Physiol. 1982;333:343–66. doi: 10.1113/jphysiol.1982.sp014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Belloc JP, Mandel JL. The FMR-1 protein is cytoplasmic most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genetics. 1993;4:335–40. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Anderson CM, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: evidence for precocious magnocellular development? Vis Res. 1999;39:3223–29. doi: 10.1016/s0042-6989(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Bosworth RG, McCleery JP. Effects of gestational length, gender, postnatal age, and birth order on visual contrast sensitivity in infants. J Vis. 2009;9:1–21. doi: 10.1167/9.10.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi H, Koga T, Shinohara K. 18-Month-olds can perceive Mooney faces. Neurosci Res. 2009;64:317–22. doi: 10.1016/j.neures.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the Bootstrap. London: Chapman and Hall; 1993. [Google Scholar]
- Farzin F, Rivera SM. Dynamic object representations in infants with and without fragile X syndrome. Front Hum Neurosci. 2010 doi: 10.3389/neuro.09.012.2010. doi:10.3389/neuro.3309.3012.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Whitney D. Holistic crowding of Mooney faces. J Vis. 2009;9:1–15. doi: 10.1167/9.6.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Whitney D. Spatial resolution of conscious visual perception in infants. Psychol Sci. 2010;21:1502–9. doi: 10.1177/0956797610382787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Whitney D. Time crawls: the temporal resolution of infant visual attention. Psychol Sci. 2011;22:1004–1010. doi: 10.1177/0956797611413291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Whitney D, Hagerman RJ, Rivera SM. Contrast detection in infants with fragile X syndrome. Vis Res. 2008;48:1471–8. doi: 10.1016/j.visres.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, Hagerman PJ, Tassone F. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn. 2009;11:324–9. doi: 10.2353/jmoldx.2009.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Jemel B, Fiori N, Chaby L, Renault B. Electrophysiological correlates of facial decisions: insights from upright and upside-down Mooney-face perception. Cognit Brain Res. 2005;24:663–73. doi: 10.1016/j.cogbrainres.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Graham NVS. Visual pattern analyzers. New York: Oxford University Press; 1989. [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA. 2001a;98:7101–6. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA. 2001b;98:7101–6. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarrenger B, Lepore F, Lippe S, Labrosse M, Guillemot JP, Roy M. Magnocellular and parvocellular developmental course in infants during the first year of life. Documenta Opthalmol. 2003;107:225–33. doi: 10.1023/b:doop.0000005331.66114.05. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–7. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proc Natl Acad Sci USA. 2010;20:9335–9. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe AO. Seeing slow and seeing fast: two limits on perception. Trends Cognit Sci. 2009;13:216–21. doi: 10.1016/j.tics.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Intriligator J, Cavanagh P. The spatial resolution of visual attention. Cognit Psychol. 2001;43:171–216. doi: 10.1006/cogp.2001.0755. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Weiler IJ, Beckel-Mitchener A, Greenough WT. Brain structure and functions of FMR1 protein. In: Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: diagnosis, treatment and research. 3rd edn. Baltimore: The Johns Hopkins University Press; 2002. pp. 191–205. [Google Scholar]
- Iwahashi C, Tassone F, Hagerman RJ, Yasui D, Parrott G, Nguyen D, et al. A quantitative ELISA assay for the fragile X mental retardation 1 protein. J Mol Diagn. 2009;11:281–9. doi: 10.2353/jmoldx.2009.080118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genetics. 2000;9:901–8. doi: 10.1093/hmg/9.6.901. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci. 1986;83:2755–7. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemelmacher-Shlizerman I, Basri R, Nadler B. 3D shape reconstruction of Mooney faces. In: Paper presented at the IEEE Conference on Computer Vision and Pattern Recognition, June, Enchorage, AK, 2008. [Google Scholar]
- Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, et al. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004a;63:1634–9. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Zangenehpour S, Mullen KT, Holden JJ, et al. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain. 2004b;127(Pt 3):591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- Latinus M, Taylor MJ. Holistic processing of faces; learning effects with Mooney faces. J Cognit Neurosci. 2005;17:1316–27. doi: 10.1162/0898929055002490. [DOI] [PubMed] [Google Scholar]
- Leo I, Simion F. Newborns' Mooney-face perception. Infancy. 2009;14:641–53. doi: 10.1080/15250000903264047. [DOI] [PubMed] [Google Scholar]
- Levi DM. Crowding-An essential bottleneck for object recognition: a mini-review. Vis Res. 2008;48:635–54. doi: 10.1016/j.visres.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins R, Hay DA, Gedeon AK, Mulley JC, Sutherland GR. Genotype–phenotype relationships in fragile X syndrome: a family study. Am J Hum Genetics. 1993;53:1064–73. [PMC free article] [PubMed] [Google Scholar]
- Louie EG, Bressler DW, Whitney D. Holistic crowding: selective interference between configural representations of faces in crowded scenes. J Vis. 2007;7:1–11. doi: 10.1167/7.2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P, Näsänen R, Rovamo J, Melmoth D. Identification of facial images in peripheral vision. Vis Res. 2001;41:599–610. doi: 10.1016/s0042-6989(00)00259-5. [DOI] [PubMed] [Google Scholar]
- Mäkelä P, Whitaker D, Rovamo J. Modelling of orientation discrimination across the visual field. Vis Res. 1993;33:723–30. doi: 10.1016/0042-6989(93)90192-y. [DOI] [PubMed] [Google Scholar]
- Martelli M, Majaj NJ, Pelli DG. Are faces processed like words? A diagnostic test for recognition by parts. J Vis. 2005;5:58–70. doi: 10.1167/5.1.6. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL. Visual outcomes after infantile cataract. In: Simons K, editor. Early visual development: normal and abnormal. New York: Oxford University Press; 1993. pp. 454–84. [Google Scholar]
- Mazzocco MMM, Bhatia NS, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner Syndrome. Child Neuropsychol. 2006;12:87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- McKone E. Isolating the special component of face recognition: peripheral identification and a Mooney face. J Exp Psychol Learn Mem Cogn. 2004;30:181–97. doi: 10.1037/0278-7393.30.1.181. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Ann Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Mooney CM. Age in the development of closure ability in children. Can J Psychol. 1957;11:216–26. doi: 10.1037/h0083717. [DOI] [PubMed] [Google Scholar]
- Moore C, Cavanagh P. Recovery of 3D volume from 2-tone images of novel objects. Cognition. 1998;67:45–71. doi: 10.1016/s0010-0277(98)00014-6. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Service Inc; 1995. [Google Scholar]
- Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38:1261–70. doi: 10.1016/s0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. J Vis. 2004;4:1136–9. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Tillman KA. The uncrowded window of object recognition. Nat Neurosci. 2008;11:1120–35. doi: 10.1038/nn.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Freund LS, Baumgardner TL, Abrams MT, Denckla MB. Contribution of the FMR1 gene mutation to human intellectual dysfunction. Nat Genetics. 1995;11:331–4. doi: 10.1038/ng1195-331. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Heitz D, Tarleton J, MacPherson J, Malmgren H, Dahl N, et al. A multicenter study on genotype–phenotype correlations in the fragile X syndrome, using direct diagnosis with probe StB12.3: the first 2,253 cases. Am J Hum Genet. 1994;55:225–37. [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J. Delineation of early attentional control difficulties in fragile X syndrome: focus on neurocomputational mechanisms. Neuropsychologia. 2007;45:1889–98. doi: 10.1016/j.neuropsychologia.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Dev Sci. 2004;7:116–30. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Scerif G, Karmiloff-Smith A, Campos R, Elsabbagh M, Driver J, Cornish K. To look or not to look? Typical and atypical development of oculomotor control. J Cognit Neurosci. 2005;17:591–604. doi: 10.1162/0898929053467523. [DOI] [PubMed] [Google Scholar]
- Schneider A, Hagerman RJ, Hessl D. Fragile X syndrome- from genes to cognition. Dev Disabil Res Rev. 2009;15:333–42. doi: 10.1002/ddrr.80. [DOI] [PubMed] [Google Scholar]
- Scholl BJ. Objects and attention: the state of the art. Cognition. 2001;80:1–46. doi: 10.1016/s0010-0277(00)00152-9. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position displacement, not velocity, is the cue to motion detection of second-order stimuli. Vis Res. 1998;38:3569–82. doi: 10.1016/s0042-6989(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Quart J Exp Psychol A. 1993;46:225–45. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. FMR1 mRNA in carrier males: a new mechanism of involvement in fragile X syndrome. Am J Hum Genetics. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy SP, Cavanagh P. The extent of crowding in peripheral vision does not scale with target size. Vis Res. 2002;42:2357–69. doi: 10.1016/s0042-6989(02)00197-9. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Verstraten FAJ, Cavanagh P, Labianca A. Limits of attentive tracking reveal temporal properties of attention. Vis Res. 2000;40:3651–64. doi: 10.1016/s0042-6989(00)00213-3. [DOI] [PubMed] [Google Scholar]
- Whitney D, Levi DM. Visual crowding: a fundamental limit on conscious perception and object recognition. Trends Cognit Sci. 2011;15:160–168. doi: 10.1016/j.tics.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001;63:1314–29. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Yin RK. Looking at upside-down faces. J Exp Psychol. 1969;81:141–5. [Google Scholar]