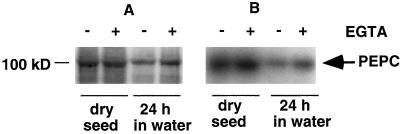
Figure 4.
BDA PEPC-PK activity from dry and soaked (24 h) whole seeds. BDA PEPC-PK from aleurone endosperm was isolated chromatographically, and in vitro phosphorylation assays were performed in the presence of exogenous, immunopurified C4 PEPC from sorghum (0.2 unit of PEPC), BDA-purified proteins from aleurone (20 μL), and the other components of the reconstituted phosphorylation reaction in the presence (+) or absence (−) of 1 mm EGTA. Radiolabeled proteins were resolved by SDS-PAGE (10% acrylamide) and detected by autoradiography. A, Coomassie blue-stained gel. B, Corresponding autoradiograph.