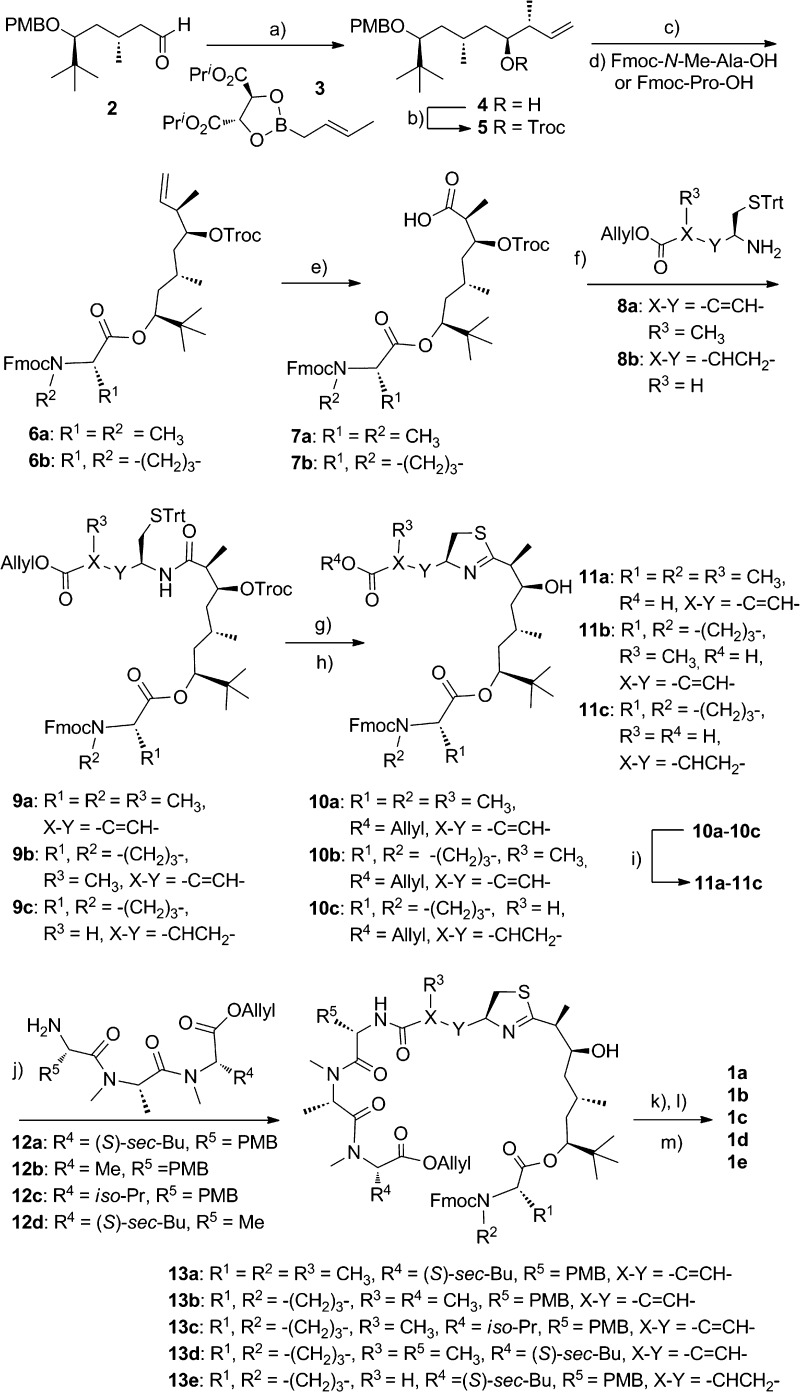
Scheme 1. Preparation of the Analogues of Apratoxin A.
Reagents and conditions: (a) MS 4 Å, toluene, −78 °C. (b) TrocCl, DMAP, pyridine, CH2Cl2. (c) DDQ, CH2Cl2–H2O. (d) Cl3C6H2COCl, DIEA, THF, DMAP, toluene. (e) OsO4, oxone, NaIO4. (f) HATU, DIEA, CH2Cl2. (g) Ph3P(O), Tf2O, CH2Cl2, 0 °C. (h) Zn, NH4OAc, THF. (i) Pd(PPh3)4, N-methylaniline, THF. (j) HATU, DIEA, CH2Cl2. (k) Pd(PPh3)4, N-methylaniline, THF. (l) Et2NH, MeCN. (m) HATU, DIEA, CH2Cl2. Troc, 2,2,2-trichloroethoxycarbonyl; DMAP, 4-(dimethylamino)pyridine; DDQ, 2,3-dichloro-4,5-dicyanobenzoquinone; Fmoc, 9-fluorenylmethoxycarbonyl; Trt, triphenylmethyl; HATU, O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate; DIEA, diisopropylethylamine; and Tf, trifluoromethylsulfonyl.