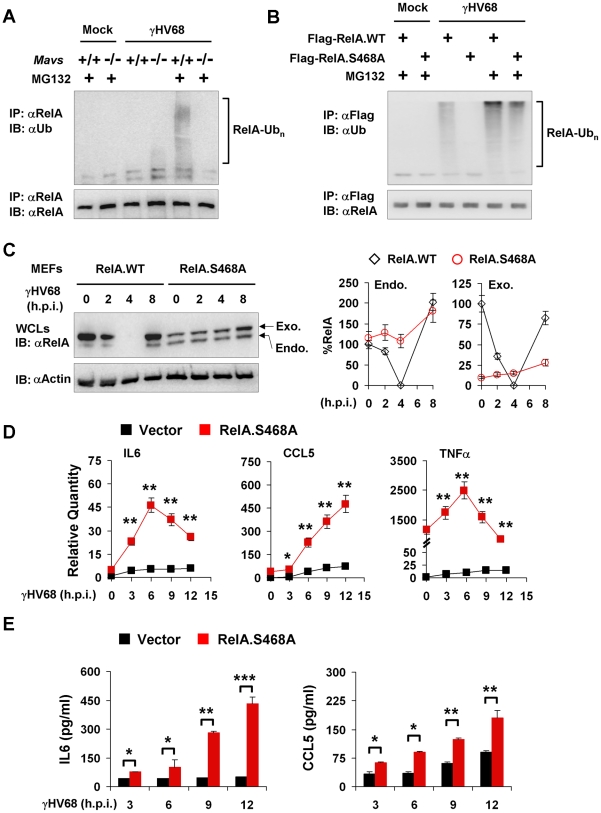
Figure 8. RelA phosphorylation at Serine 468 is critical for γHV68-induced RelA degradation.
Mouse embryonic fibroblasts (MEFs) were infected with γHV68 K3/GFP at an MOI of 20 (A, B, and C) or 5 (D and E). (A) At 2 hours post-infection, mock- or γHV68-infected Mavs +/+ and Mavs −/− MEFs were treated with MG132 (20 µM) for an hour or left un-treated. Endogenous RelA was precipitated and analyzed by immunoblot with indicated antibodies. (B) Mavs +/+ MEFs stably expressing Flag-tagged wild-type RelA (RelA.WT) or the RelA.S468A variant were established as described in Materials and Methods. MEFs were infected with γHV68 and treated with MG132 (20 µM) as in (A). Exogenous RelA was precipitated with anti-Flag antibody and analyzed by immunoblot with indicated antibodies. (C) Whole cell lysates (WCLs) from γHV68-infected Mavs +/+ MEFs stably expressing wild-type RelA or the RelA.S468A variant were analyzed by immunoblot. Endo., endogenous RelA; Exo., exogenous RelA. Relative quantity of RelA was normalized to β-actin. Percentage of RelA protein was calculated in reference to that of uninfected MEFs (RelA) (right). (D and E) Control (Vector) or RelA.S468A-expressing Mavs +/+ MEFs were infected with γHV68 for indicated time. (D) Cytokine mRNA levels were analyzed by real-time PCR and normalized to that of β-actin. (E) Cytokine levels in the supernatant were determined by ELISA. Data are presented as the mean ± SEM of three independent experiments. The statistical significance: *, P<0.05; **, P<0.02; ***, P<0.005. See also Figure S12.