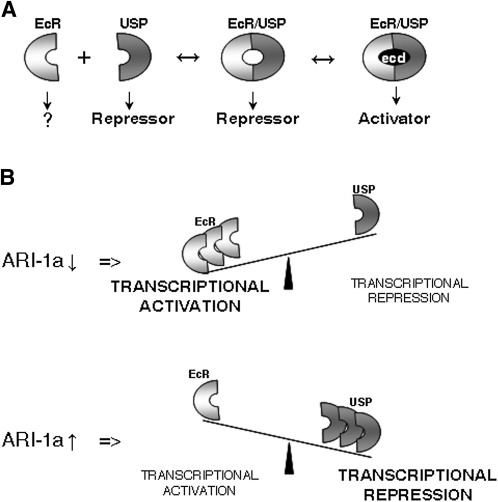
Figure 7 .
Summary of results and functional proposal. (A) It is known that EcRA and USP need to dimerize to reconstitute a nuclear transcriptional activator. The isolated monomers are located in the cytoplasm (EcRA) or in the nucleus (USP) where the latter behaves as a repressor (Gwozdz et al. 2007). As previously suggested, the two monomers could bind generating unliganded complex units, which would act as a transcriptional repressor (Schubiger et al. 2005). At metamorphosis, ecdysone (ecd) titers increase, and the complex can become a transcriptional activator upon ecd binding. (B) On the basis of the data reported here, we propose that the equilibrium between EcRA and USP proteins can be displaced as a function of ARI-1a activity. If ARI-1a is diminished (RNAi) or absent (ari-1a2), the balance would displace in favor of EcRA, and the predominant transcriptional effect upon the tested genes, including EcR, would be activation. By contrast, if ARI-1a increases (tub-Gal4LL7 > UAS-ari-1a), the equilibrium would move in the opposite direction, and transcriptional repression would dominate most of the tested genes. This functional proposal is a generalization that does not take into account the possible tissue specificities in terms of stoichiometry of the proteins involved.