Figure 3.
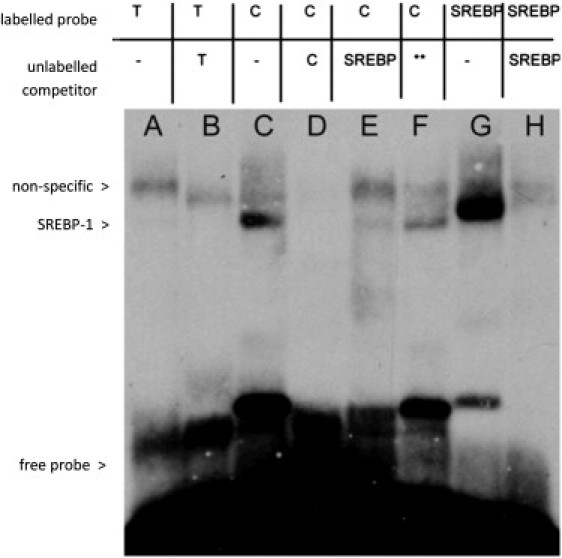
Electrophoretic Mobility Shift Assay Demonstrating Allele-Specific Transcription Factor Binding to rs1466535
Lane A: binding of nuclear extract to labeled oligonucleotide containing rs1466535 T allele; lane B: T-allele binding with addition of excess unlabeled T-allele DNA competitor. The remaining band indicates a nonspecific protein-DNA interaction. Lane C: binding of nuclear extract to labeled oligonucleotide containing C allele. Lane D: C-allele binding with addition of excess unlabeled C-allele probe. Elimination of bands present in lane C indicates the binding of a specific protein. Lane E: C-allele-labeled oligonucleotide with addition of excess unlabeled fSREBP-1 (a transcription factor known to play an important role in LRP1 transcription) DNA competitor. The reduction in intensity of the lower band indicates that this binding is likely to be due to SREBP-1. Lane F: C-allele-labeled oligonucleotide with addition of excess non-SREBP-1-binding oligonucleotide competitor (sequence available on request) as a negative control. Lane G: binding of nuclear extract to labeled SREBP-1 consensus sequence, the lower band migrates with the putative SREBP-1 bound to the C allele. Lane H: SREBP-1 consensus sequence with the addition of excess SREBP-1 unlabeled competitor DNA. The lower band is eliminated, indicating this band represents the DNA-SREBP-1 complex. Together, these results suggest that rs1466535[T] removes a binding site for SREBP-1 in vitro.
