Abstract
For more than 60 years, the chemical induction of tumors in mouse skin has been used to study mechanisms of epithelial carcinogenesis and evaluate modifying factors. In the traditional two-stage skin carcinogenesis model, initiation is accomplished by the application of a subcarcinogenic dose of a carcinogen. Subsequently, tumor development is elicited by repeated treatment with a tumor promoting agent. The initiation protocol can be completed within 1–3 hours depending on the number of mice used, while the promotion phase requires twice weekly treatments (1–2 hours) and once weekly tumor palpation (1–2 hours) for the duration of the study. A highly reproducible papilloma burden is expected within 10–20 weeks with progression of a portion of the tumors to squamous cell carcinomas within 20–50 weeks. In contrast to complete skin carcinogenesis, the two-stage model allows for greater yield of premalignant lesions as well as separation of the initiation and promotion phases.
INTRODUCTION
Historical Perspective
The mouse skin model of multi-stage chemical carcinogenesis represents one of the best established in vivo models for the study of the sequential and stepwise development of tumors. In addition, this model can be used to evaluate both novel skin cancer prevention strategies and the impact of genetic background and genetic manipulation on tumor initiation, promotion, and progression. The multistage nature of the carcinogenic process was first clearly demonstrated in the mouse skin modelreviewed in 1. In the 1920s it was noted that wounding of mouse skin that had previously been treated with carcinogenic tar could lead to the appearance of tumors. These findings suggested a role for cell proliferation and hyperplasia in a multi-step evolution of cancer, and ultimately, led to the development of the two-stage protocol for mouse skin tumorigenesis (Fig. 1). From these seminal studies to the present, mouse skin carcinogenesis has become one of the most extensively analyzed rodent models of chemically-induced cancerreviewed in 2,3–10. Studies in this model have yielded, and continue to yield, insight into the fundamental biology of cancer, and much of our understanding of the multi-stage nature of epithelial cancers is rooted in the analysis of chemically-induced skin tumors in mice.
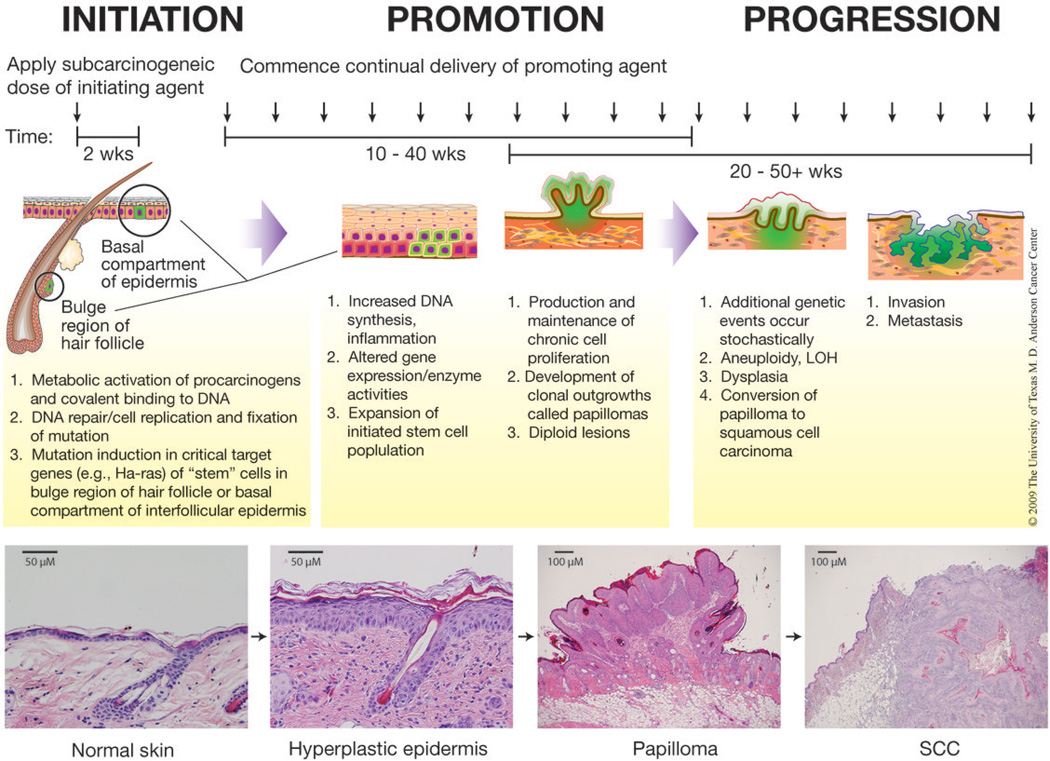
Figure 1. Two-stage model of skin carcinogenesis in mice.
During initiation, topical application of a sub-carcinogenic dose of a mutating agent induces mutations in target genes in keratinocyte stem cells. Repeated topical application of a promoting agent begins two weeks after initiation and continues for the duration of the study. Papillomas begin to arise after approximately 6–12 weeks of promotion and a fraction begin to convert to SCC after approximately 20 weeks. Representative H&E stained sections of normal skin, hyperplastic skin, a papilloma, and a SCC are presented. All mice were handled in accordance with institutional and national regulations. This figure is a modification of a previously published figure133.
“Complete” or “two-stage” carcinogenesis protocols have been developed for the study of skin tumors in mice (i.e., tumor incidence, latency, multiplicity, and progression). In complete carcinogenesis protocols, tumor development occurs after either the administration of a single high dose (or repeated applications of a lower dose) of a carcinogen or by continuous exposure to ultraviolet (UV) light. Additional treatments with promoting agents are not required for tumor development, indicating that both the initiating and promoting components are present during complete carcinogenesis. However, the interpretation of complete carcinogenesis studies is complicated by the inability to distinguish events or effects related to the tumor initiation versus tumor promotion stages. In two-stage skin carcinogenesis experiments, initiation occurs following a single subcarcinogenic dose of a carcinogen such as 7,12-dimethylbenz[a]-anthracene (DMBA) (Fig. 1). This event is irreversible; however, no visible tumors will appear until ‘promoted’ by the repeated application of a tumor promoting agent such as the phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA). Thus, in two-stage skin carcinogenesis experiments, the initiation and promotion stages can be distinctly separated both operationally and mechanistically.
An additional advantage of the two-stage skin carcinogenesis model is that tumor development can be conveniently monitored visually throughout the life span of the mouse; tissue harvest and pathological analysis are only necessary at the termination of the study or when an animal requires sacrifice. Since the tumor response is highly reproducible, the efficacy of chemopreventive agents or the impact of dietary manipulation can be assessed11–13. Additionally, the role of various genes and cell-signaling pathways can be explored in this model through the use of genetically engineered mouse models (GEMMs) as well as small molecule inhibitors14–23. A number of established short-term markers of skin carcinogenesis aid in determining the stage at which modifying factors affect tumorigenesis and give clues as to the molecular mechanisms involvedreviewed in 1,24. In light of the extensive characterization of this model as well as its versatility, two-stage skin carcinogenesis in mice continues to serve as a useful model of human cancers of epithelial originreviewed in 2,25.
Description of the Model
During the first stage of chemically-induced skin carcinogenesis, referred to as ‘initiation’, key genes in epidermal keratinocytes acquire mutations as a result of exposure to a chemical mutagen. Currently, the most frequently utilized initiating agent is the polycyclic aromatic hydrocarbon, DMBA, but additional agents can serve as chemical initiators (Table 1). DMBA is most often applied topically, although systemic exposure is also effective26. The Hras1 gene appears to be a primary target gene for the initiation stage, although mutations in Kras have been demonstrated in lesions initiated with DMBA and N-methyl-N’-nitro-N-nitrosoguanidine (MNNG), and Nras mutations have been observed in lesions initiated with UV light, (Table 1)27–29. Activating mutations in Hras1 can be detected in the epidermis as early as 3–4 weeks following treatment with DMBA30 and are observed in the majority of papillomas that develop initially following tumor promoter treatment31. While DMBA predominantly induces an A → T (182) transversion in codon 61 of the Hras1 gene, various other initiating agents each produce a unique spectrum of activating Hras1 mutations32. The observation that skin tumors are induced in TG.AC mice (transgenic mice that express a v-Ha-ras under the control of the zeta-globin promoter) following promotion by a variety of agents without prior treatment with an initiating agent, supports mutation of Hras1 as a critical event in skin carcinogenesis33. Keratinocyte stem cells, which are primarily found at the base of epidermal proliferative units in the interfollicular epidermis and in the bulge region of the hair follicles, are believed to be the primary cellular targets of the initiation stage34.
Table 1.
Examples of chemical or physical agents that can serve as initiating or promoting agents and their primary molecular target or event.
| Initiating agents | Genetic Targeta |
Promoting agents | Initial molecular target or event associated with tumor promotion |
|---|---|---|---|
| DMBA | Hras1, Kras | TPA | Protein Kinase C |
| Benzo[a]pyrene (B[a]P) | Hras1 | Telocidin | Protein Kinase C |
| MNNG | Hras1, Kras | Okadaic acid | Protein Phosphatases -1 and -2A |
| N-methyl-N-nitrosourea (MNU) | Hras1 | Chrysarobin | Generates oxidative stress |
| Bis(chloromethyl)ether | Unknown | Benzoyl peroxide | Generates oxidative stress |
| Ultraviolet radiation | Tp53, Nras | Ultraviolet radiation | Protein Kinase C, EGFR |
| Cisplatinum | Hras1 | Wounding | Stimluation of EGF receptor |
| β-propiolactone | Hras1 |
Primary target based on use of TPA as the promoting agent or, in the case of UV, the use of a complete carcinogenesis regimen
Following the initiation stage, the population of mutated cells is promoted to clonally expand during the second stage, referred to as “promotion”. Tumor promotion is elicited by the repeated topical application of chemical agents or wounding that leads to sustained epidermal hyperplasia evidenced by an increase in the number of nucleated cell layers and an overall increase in thickness of the epidermisreviewed in 1,2–10,35. During epidermal hyperplasia, initiated cells are believed to have a growth advantage over neighboring cells allowing for their selective expansion36–38. The stimulation of growth of initiated cells may be a direct effect of the tumor promoter on these cells and/or occur through indirect effects due to loss of cell populations1,8,39. The end result of the promotion stage is the development of clonal outgrowths of the skin called papillomasreviewed in 1,2–10,35. Papillomas consist of a stromal core surrounded by hyperplastic epidermis (Fig. 1). Promoting agents, which are structurally diverse as well as mechanistically diverse in action (Table 1), stimulate cell signaling, increase production of growth factors, and generate oxidative stress and tissue inflammationreviewed in 1. Therefore, short-term markers of tumor promotion include increased epidermal thickness, proliferation of basal keratinocytes, increased DNA synthesis, and inflammatory cell infiltrationreviewed in 1,2–10,35.
Papillomas generated during two-stage skin carcinogenesis protocols may progress to invasive squamous cell carcinomas (SCC) as early as 20 weeks after treatment with the promoting agent begins (Fig. 1). The frequency of malignant conversion is dependent on genetic background; for instance, as few as 1–10% of papillomas progress to SCC in SENCAR or BALB/c mice while up to 50% of papillomas that develop in skin of FVB mice may convert40–42. This is also highly dependent on doses of initiator and promoter, which influence papilloma burden43,44. Further progression can lead to formation of spindle cell carcinomas, although this is a relatively rare event35. During the conversion process, progressive chromosomal abnormalities occur independent of continued treatment with tumor promoting agents. The tumors become aneuploid ~30–40 weeks after the initiation protocol begins45–47. In this regard, the conversion of papillomas to SCCs is associated with trisomies of chromosomes 6 and 7 as well as mutations in Trp5348,49. SCCs are downward invading lesions that are highly vascularized. Numerous gene expression changes are present, including those associated with epithelial-mesenchymal transition (EMT)1,50–52. Approximately 15–35% of mice with one or more carcinomas will also have metastases to organs such as lung or lymph nodes, and the frequency of metastasis appears to be under genetic control53.
Susceptibility to two-stage skin carcinogenesis in mice is known to be highly dependent on genetic background (Table 2)reviewed in 1. Early studies by Boutwell supported the hypothesis that specific genes modify susceptibility to two-stage skin carcinogenesis54. Different stocks and strains of mice do not significantly differ in the capacity of epidermis to metabolize initiating agents (e.g., polycyclic aromatic hydrocarbons such as DMBA). In addition, the formation and removal of covalent DNA-adducts during skin tumor initiation appears to be similar. Thus, the primary genetic determinants of susceptibility to two-stage skin carcinogenesis likely lie in response to tumor promotionreviewed in 55. Supporting this hypothesis, mice initiated with direct acting carcinogens, such as MNNG or UV-light, show the same distribution in susceptibility to two-stage epidermal carcinogenesisreviewed in 55. These results indicate that the strain-dependent response to two-stage skin carcinogenesis is not due to differences in metabolic activation of the initiating agent; rather, it is most likely due to variation in the effects elicited by treatment with promoting agent.
Table 2.
Expected tumor response of various mouse stocks and strains subjected to two-stage skin carcinogenesis using various initiating and promoting agents
| Initiator Dose | Promoter Dose | Mouse Strain | % Papilloma Incidence |
Papillomas/ Mouse |
Reference | |
|---|---|---|---|---|---|---|
| DMBA/TPAa | ||||||
| 25 nmol | 1.7 nmol | SENCAR | 100 | 21.7 | 125 | |
| 0.1–100 nmol | 8.5 nmol | SENCAR | 20–100 | 0.6–24 | 126 | |
| 0.1–100 nmol | 8.5 nmol | CD-1 | 0–90 | 0–5.6 | 126 | |
| 20–100 nmol | 1–5 µg (1.6–8.1 nmol) | 129/SvEv | 100 | 10 | 127 | |
| 10 nmol | 6.8 nmol | DBA/2 | 94 | 5.7 | 128 | |
| 400 nmol | 1.7–6.8 nmol | DBA/2 | 15–100 | 0.25–8.67 | 129 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | DBA/2 | 83 | 3.6 | 130 | |
| 100 µg (390 nmol) | 2.5 µg (4 nmol) | FVB | 97 | 12.7 | 131 | |
| 97.4 nmol | 32.4 nmol | FVB | 100 | 32.5 | 59 | |
| 100 nmol | 6.8–13.6 nmol | FVB | 100 | 20–25 | 132 | |
| 100 nmol | 3.4–6.8 nmol | FVB | 54–100 | 1–10 | 20 | |
| 25 nmol | 5 nmol | FVB | 100 | 10.9 | 23 | |
| 97.4 nmol | 32.4 nmol | PWK | 0 | 0 | 59 | |
| 10 nmol | 6.8 nmol | C57BL/6 | 4 | 0.04 | 128 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | C57BL/6 | 42 | 2.2 | 130 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | I/LnJ | 100 | 4.3 | 130 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | HRS | 83 | 4.6 | 130 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | BALB/c | 83 | 4.4 | 130 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | C3H/He | 67 | 5.1 | 130 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | A/J | 64 | 2.2 | 130 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | SJL | 45 | 1.9 | 130 | |
| 25 µg (97.5 nmol) | 4 µg (6.5 nmol) | CBA | 45 | 1.6 | 130 | |
| 400 nmol | 1.7–10 nmol | (C57BL6 × DBA/2)F1 hybrid | 0–100 | 0–11.8 | 129 | |
| DMBA/TPAb | ||||||
| 5–100 µg (19.5–390 nmol) | 0.5–5 µg (0.8–8 nmol) | FVB | 20–100 | 0–11.8 | 42 | |
| 25 µg (97.5 nmol) | 2 µg (3.2 nmol) | SENCAR | 100 | 18.8 | 42 | |
| 25 µg (97.5 nmol) | 2 µg (3.2 nmol) | CD-1 | 53 | 2.5 | 42 | |
| 25 µg (97.5 nmol) | 2 µg (3.2 nmol) | C57BL/6 | 0 | 0 | 42 | |
| 25 µg (97.5 nmol) | 2 µg (3.2 nmol) | BALB/c | 17 | < 1 | 42 | |
| DMBA/Chrysarobinb | ||||||
| 25 nmol | 10–100 nmol | SENCAR | 20–100 | 0.9–9.2 | 66 | |
| 25 nmol | 10–100 nmol | SSIn | 4–79 | 0.04–1.83 | 66 | |
| 25 nmol | 100–880 nmol | DBA/2 | 33–58 | 0.7–1.5 | 66 | |
| 25 nmol | 100–880 nmol | C57BL/6 | 0–46 | 0–0.5 | 66 | |
| DMBA/Benzoyl peroxidea | ||||||
| 25 nmol | 5–20 mg | SENCAR | 60–88 | 4.4–.7.2 | 66 | |
| 25 nmol | 5–20 mg | SSIn | 96–100 | 11.7–14.3 | 66 | |
| 25 nmol | 10–40 mg | DBA/2 | 35–44 | 0.5–0.6 | 66 | |
| 25 nmol | 10–40 mg | C57BL/6 | 18–39 | 0.4–0.6 | 66 | |
| DMBA/Telocidina | ||||||
| 25 nmol | 0.43–1.7 nmol | SENCAR | 4–31 | 0.24–.088 | 67 | |
| 25 nmol | 0.85–3.4 nmol | C57BL/6 | 4–15 | 0.04–0.19 | 67 | |
| MNNG/TPAa | ||||||
| 2.5 µmol | 6.8 nmol | SENCAR | 83 | 3.3 | 94 | |
| 2.5 µmol | 1.7–10 nmol | CD-1 | 0–80 | 0–2.2 | 66 | |
| 2.5 µmol | 17.0 nmol | C3H/He | 97 | 1.6 | 94 | |
| 2.5 µmol | 1.7–13.6 nmol | DBA/2 | 33–95 | 0.9–1.65 | 129 | |
| 2.5 µmol | 3.4–17.0 nmol | C57BL/6 | 0–10 | 0–0.1 | 129 | |
| 2.5 µmol | 1.7–13.6 nmol | (C57BL6 × DBA/2)F1 hybrid | 5–90 | 0.5–1.3 | 129 | |
| MNNG/Chrysarobinb | ||||||
| 2.5 µmol | 25–440 nmol | SENCAR | 48–90 | 0.9–4.5 | 66 | |
| 2.5 µmol | 440–880 nmol | SSIn | 8–21 | 0.08–0.2 | 66 | |
| 2.5 µmol | 25–440 nmol | CD-1 | 0–5 | 0–0.05 | 66 | |
| 2.5 µmol | 440–880 nmol | DBA/2 | 25 | 0.25–0.32 | 66 | |
| 2.5 µmol | 440–880 nmol | C57BL/6 | 21–24 | 0.25 | 66 | |
| MNNG/Benzoyl Peroxidea | ||||||
| 2.5 µmol | 10–20 mg | SENCAR | 20–52 | 0.5–2.1 | 66 | |
| 2.5 µmol | 10–20 mg | SSIn | 83–92 | 2.4–2.6 | 66 | |
Promoter applied twice weekly
Promoter applied once weekly
The majority of studies assessing tumor promotion susceptibility in mice have used phorbol esters such as TPA. The distribution pattern for sensitivity to tumor promotion by TPA (SENCAR > DBA/2 ≥ CD-1 > C3H/He >> C57BL/6) has been well documentedreviewed in 55. A number of chromosomal loci underlying this complex trait have been identified in various stocks and strains of mice56–63. A similar distribution pattern for the sensitivity to different classes of promoting agents suggests that common genetic factors modify the response to tumor promotion by different classes of promoting agents64. For example, the distribution pattern for sensitivity to skin tumor promotion by chrysarobin, benzoyl peroxide, and full thickness skin wounding is SENCAR > DBA/2 > C57BL/664–66. Because of these genetic differences in response to skin tumor promoters, careful attention needs to be paid to the genetic background of the mouse strain being studied when selecting a dose of the promoting agent. For example, a higher dose of TPA and a different treatment regimen (i.e., three times weekly application) may be used when studying C57BL/6 mice compared with SENCAR mice67. As will be discussed, the choice of mouse strain and doses of initiating and promoting agents as well as the timing of interventions are critical design options that affect the outcome and interpretation of two-stage skin carcinogenesis studies in mice.
Potential Applications of the Model
Multiple lines of evidence suggest that epithelial cancers in humans are the result of a multistage process68,69. Cancer is widely believed to arise from the expansion of stem cell populations targeted during a DNA-mutating event such as carcinogen exposure70. In the case of colon cancer, the accumulation of numerous genetic lesions in an increasingly aberrant subset of tumor cells reflects the multiple steps required for epithelial carcinogenesis, and these genetic changes are reflected in progressive histopathological changes from hyperplasia to adenoma to true carcinomas71–73. The two-stage skin carcinogenesis model in mice recapitulates features of multi-stage carcinogenesis in humans. For example, similar to a number of solid tumors in humans, it appears that the occurrence of activating mutations within stem cell niches is the first step in a cascade of events leading to tumor formation in this model74,75. Additionally, there are a number of similarities to human cancers at the genetic or molecular level, including activating mutations in ras family members, activation of the epidermal growth factor receptor (EGFR), activation of Stat3 and Akt-mediated signaling pathways, elevated expression of transforming growth factor β1, and, at later stages, Tp53 mutations2. Likewise, the two-stage skin carcinogenesis protocol is a good model for human cancers because humans are typically exposed to multiple low doses of both carcinogens and promoting agents76. The long latency associated with most human cancers also strongly supports a promotional component for tumor development77,78. Therefore, this extensively characterized model can be utilized to study the mechanistic basis of human epithelial cancers.
Recent advances in the generation of GEMMs have provided valuable tools in furthering our understanding of the carcinogenic process. Our laboratory and others have used GEMMs in the two-stage skin carcinogenesis protocol to investigate the roles of specific genes in epithelial carcinogenesis16–23. Importantly, gene function can be studied in vivo and throughout the process of carcinogenesis. Also, the development of methods for targeting genetic modifications to the skin has allowed gene expression or gene deletion in specific compartments of the skin, avoiding complications such as embryonic lethality or systemic effects79. Two-stage skin carcinogenesis studies using GEMMs can reveal the roles of potential cancer risk modifier genes, proto-oncogenes, and tumor suppressor genes in tumor initiation, promotion, and progression. In addition, chemical inhibitors may also be used to test the role of specific signaling pathways during carcinogenesis. For example, recent studies from our lab using a PI3K inhibitor, LY294002, in a transgenic mouse model overexpressing human IGF-1, underscored the role of PI3 Kinase and Akt-mediated signaling in epithelial carcinogenesis14.
The mouse multi-stage skin carcinogenesis model is particularly suited for evaluating the effects of dietary factors/dietary manipulations and other chemicals (both natural and synthetic) on tumor development. Potential inhibitors of carcinogenesis can be evaluated for their effects on initiation, promotion, and/or progression11–13. For example, studies from our lab showed that delivery of the citrus coumarin, isopimpinellin, prior to DMBA exposure significantly inhibited tumor initiation80. In contrast, sulforaphane was most effective at inhibiting the promotion stage81 while silymarin was effective at blocking tumor formation as well as inducing regression of established tumors depending on the sequence of delivery82. Although not yet widely examined, the possibility exists that this model may also be useful for evaluating the efficacy of therapeutic agents. Recent results showing regression of papillomas injected with a Stat3 decoy oligonucleotide21 or treatment of existing tumors with rapamycin15 support the feasibility of this application of the model. Importantly, topical application of chemopreventive agents as well as initiating and promoting agents precludes or reduces systemic effects.
The effect of dietary manipulation on carcinogenesis can also be studied in this model system. Since calorie restriction in known to inhibit the tumor promotion phase, this model is ideally suited for investigating the mechanistic basis for the link between negative energy balance and cancer risk54,83. Tissue specific alterations, as well as systemic effects of altered energy balance, may be examined84,85. In any chemoprevention or dietary intervention study, specific attention to study design is necessary to properly assess efficacy and draw rational conclusions.
While the two-stage skin carcinogenesis model has a variety of applications and has been extensively utilized to address of a number of questions about the fundamental biology of epithelial cancers, some limitations exist. In this protocol, mice develop primarily papillomas, of which there is no direct human equivalent; however, the SCCs that develop following malignant conversion are histologically very similar to human SCCs. Another limitation of the model is that Hras is the primary target for chemical initiation in mice, whereas Tp53 appears to be a more important target for gene mutation in human non-melanoma skin cancer86. The gene targets for initiation by chemicals in mouse skin more closely resemble those found in other human epithelial cancers (e.g., lung, colon, and pancreatic cancers)87. Additionally, the two-stage skin carcinogenesis protocol is of limited utility for studying metastasis. As is true for a number of other mouse models of cancer, the rate of metastasis of skin tumors is quite low53.
Experimental Design Considerations
The physical application of tumor initiating and promoting agents to mouse skin as well as the palpation of papillomas and SCCs is not technically challenging. As will be described, a solution of DMBA is applied to the shaved dorsal skin of mice to accomplish the initiation phase. Subsequently, TPA is applied to the skin twice weekly until the tumor response reaches a plateau. Any palpable mass greater than 1 mm in size can be considered a papilloma and recorded. Despite the technical ease of performing this protocol, the proper design and execution of a two-stage skin carcinogenesis study requires thoughtful planning and diligent attention to detail. In this section, a number of experimental design and execution considerations will be discussed.
A thorough understanding of chemically-induced carcinogenesis as well as sound hypothesis development are necessary for the proper design of two-stage skin carcinogenesis studies in mice. In developing a testable hypothesis, consideration should be given to the specific goal of the study. Is the goal to examine gain or loss of gene function or the effect of a chemopreventive agent on initiation, promotion, or progression? Existing literature concerning the role of the gene or agent can be very useful in developing a biologically plausible hypothesis. If the anticipated effect is on carcinogen metabolism, DNA repair, mutation induction, or cell survival, then more attention should be given to the initiation stage, although factors or agents that affect maintenance of genomic stability and/or cell survival may also play a role in tumor progression in this model. In this respect, examination of short-term makers for effects on tumor initiation (i.e., DNA adduct formation and carcinogen-induced apoptosis) can guide the design of tumor experiments21,80. For agents or genes that affect cell proliferation, oxidative stress, or inflammation, a role in the tumor promotion stage may be hypothesized. Agents or genes with known effects on EMT, vascularization, or cell motility may be hypothesized to affect tumor progression50,88–92. As with initiation, preliminary studies of short-term markers of tumor promotion (i.e., epidermal proliferative response or dermal inflammation) or tumor progression (i.e., keratinocyte migration or invasion) may be performed prior to the design of tumor experiments20,50,93,94. Ideally, a set of well-designed experiments addresses all stages, although practical considerations may dictate focus on only one of the stages (e.g., initiation and/or promotion).
After development of a testable hypothesis, among the most critical next decisions are the choice of initiating and promoting agents as well as the dose of each to be applied. These parameters not only dictate the conclusions that may be drawn from the study but also the anticipated tumor burden and the health and welfare of the animals. Also, the choice of initiating and promoting doses may determine whether or not the effect of a chemopreventive agent or genetic manipulation can be accurately determined. For instance, the use of very high doses of these agents may overwhelm a potential chemopreventive effect or prevent accurate assessment of tumor progression. Depending on the anticipated effect, experiments that incorporate dose-response analyses are highly recommended, wherein a range of tumor initiator or tumor promoter doses is examined or, alternatively, a range of potential inhibitor doses is used.
An additional consideration for chemoprevention (or gene modification) experiments is the timing of agent delivery. By altering the time at which chemopreventive agent is delivered or altered gene expression occurs, effects on tumor initiation, promotion or progression may be monitored. From laboratory to laboratory, variation in the preferred waiting period between the initiation and promotion stages exists; our laboratory routinely utilizes a 2-week interval. It is important to note, however, that the time following initiation can be extended for many weeks without a negative impact on tumor yieldreviewed in 9. Therefore, this waiting period can be adjusted to fit the goals of the study. For instance, when studying the effects of calorie restriction, we routinely initiate, and then place the mice on a calorie restricted diet for up to 8 weeks prior to beginning the promotion phase (unpublished data).
The choice of mouse strain and the number of mice utilized per group are additional factors to consider in designing a successful two-stage skin carcinogenesis experiment. Moreover, given the well-described variation in sensitivity to two-stage skin carcinogenesis among mouse stocks and strains, the choice of dose of the promoting agent and mouse strain are interdependent. It is important to recognize that the development of both papillomas and SCCs is under independent genetic control95. Therefore, selection of an appropriate genetic background is critical for the hypothesis to be tested. For many years, outbred CD-1 and SENCAR mice have been used for two-stage skin carcinogenesis studies96–99. In more recent years, two-stage skin carcinogenesis experiments have used a variety of genetic backgrounds due, in part, to the generation of gain and loss of function GEMMs on diverse genetic backgrounds. FVB mice have emerged as an appropriate inbred mouse strain for two-stage skin carcinogenesis studies20,42. The use of an inbred mouse strain reduces variability in tumor response, and FVB mice represent a strain that is moderately sensitive to tumor promotion by TPA, which facilitates analysis of modifying factors (both positive and negative). In some cases, it is not possible to use FVB mice or even an inbred strain. Therefore, as a guideline in these instances, recommended dosing regimens as well as expected outcomes for various mouse stocks and strains are listed in Table 2. Since repeated wounding due to fighting or irritation of the skin can act to promote skin tumor formation, aggressive mice should not be group housed. For this reason, female mice are preferred due to less aggressive behavior, which permits reduction in animal housing costs. It is often useful and even necessary to identify individual mice in each group housing by ear punch pattern, tail tattoo, or other identification methods.
In studies of GEMMs, it is important to use wild-type littermates as control mice to insure that genetic background does not confound results. It is also recommended that null alleles or transgenes be backcrossed at least 10 generations to an inbred mouse strain that is moderately sensitive to skin carcinogenesis before performing two-stage skin carcinogenesis experiments. Alternatively, a process commonly called “speed congenics” can be employed, wherein marker assisted selective breeding is used to transfer the genetic modification to an inbred background in less time, using fewer mice100. These are critical considerations in studies of bi-transgenic or inducible gene deletion models since very high levels of heterogeneity will result if transgenic mouse strains of discrepant genetic background are crossed. It is also especially important to include additional control groups in the study design when characterizing a novel GEMM or potential carcinogen. As appropriate to the goal of the study, vehicle only control groups for the initiation and/or promotion stages should be included when using either non-transgenic mice or GEMMs. These controls may reveal potential endogenous initiating or promoting activity associated with genetic modification. Sample size calculations can be performed during the experimental design phase to predict the number of mice needed per group to allow ample statistical power to compare the response of control and test groups101,102. (Additional information concerning sample size determinations can be found at http://web.ncifcrf.gov/rtp/lasp/intra/acuc/fred/animal_number.asp). Power calculations should take into account that tumor response data is not normally distributed; therefore, use of normative tests will underestimate the required sample size.
The mice used in two-stage skin carcinogenesis studies should be age- and gender-matched and then randomly assigned to treatment groups (using a random number generator). Although some variation exists, most mice are in telogen (resting phase) at 7–9 weeks of age. When possible, this is the target age for beginning a two-stage skin carcinogenesis experiment. The majority of the dorsal skin of the mice should be shaved 48 hr prior to application of the initiating agent. (Waiting 48 hours before applying the initiating agent allows any inflammation generated during shaving to dissipate.) Once shaved, the skin of the mice should be examined to determine the approximate phase of the hair cycle. The skin of FVB mice in the anagen phase of the hair cycle appears thickened and white compared to skin in the resting phase due to extended hair follicle length. Similarly, the skin of pigmented mice in anagen is much darker. In addition, mice still in anagen may display partial hair regrowth after shaving. Any mice that are not found to be in the resting phase of the hair cycle should be eliminated from the study.
Another important consideration is the choice of diet. In this regard, specific dietary constituents and chow composition may also influence tumor response. A semi-purified chow, such as AIN- 76 diet, is often preferred for two-stage skin carcinogenesis experiments since the composition of natural-ingredient chow is likely to fluctuate depending on the cultivation, harvest, and storage conditions of the plant or fish components103. Consequently, it is imperative that control and test groups have equal ability to access identical diets. Factors that affect food consumption, such as digestive tract malformations, extreme tumor burden, or unpalatable chow, may confound results. Therefore, body weight should be monitored regularly throughout the study to insure that groups maintain similar rates of weight gain.
Finally, if one of the goals of the two-stage skin carcinogenesis study is to examine the conversion of papillomas to SCCs, additional considerations are needed. To achieve the maximum number of conversion events, treatment with the tumor promoting agent should be continued for the duration of the experiment (e.g., up to 52 weeks for SCC development)43,44. Papillomas can be expected to convert to SCCs after ~20 weeks of promotion in most wild-type strains. The presence of SCC should be confirmed histologically at the conclusion of the studies; however certain macroscopic characteristics indicate the presence of SCC. Tumors with flattened and downward appearing growth as well as attachment to the underlying muscle layer can be considered SCCs. As noted above, significant variation in the rate of conversion of papillomas to SCCs exists among various stocks and strains of mouse. A genetic background that confers sensitivity to tumor progression, such as SENCAR or FVB, should be selected. Also, as is true for papilloma formation, the doses of initiating and promoting agent will determine the ultimate response43. Use of high doses is not recommended both to insure that the mice do not become morbid due to tumor burden and because the rate of conversion to SCCs is limited by papilloma burden and biological constraints of the skin44. In general, the use of excessive doses of initiating and/or promoting doses is discouraged since as the tumor burden increases, lesions may coalesce and become difficult to track.
In this article, a standard two-stage skin carcinogenesis protocol using FVB mice is presented. As noted above, FVB mice are moderately sensitive to tumor promotion by TPA and are susceptible to development of SCCs42. Therefore, relatively low doses of DMBA (25 nmol) and TPA (1.7, 3.4, and 6.8 nmol) can be utilized. Similar treatment regimens have been extensively used by numerous groups (see Table 2), demonstrating the reliability and reproducibility of this model.
MATERIALS
REAGENTS
-
Female FVB mice 7–9 weeks of age with dorsal skin in the resting phase of the hair cycle.
!CAUTION—All experiments involving animals should be undertaken in compliance with national and institutional regulations.
-
DMBA, ≥ 95% pure (25 nmol/0.2 ml acetone) (D3254, Sigma-Aldrich, St. Louis, MO)
!CAUTION—Hazardous mutagenic compound. Avoid contact.
!CRITICAL—DMBA is light sensitive.
-
TPA, ≥ 99% pure (1.7, 3.4, and 6.8 nmol/0.2 ml acetone) (P-1680, LC Laboratories, Woborn, MA)
!CAUTION—Potentially hazardous compound. Avoid contact.
-
Acetone (HPLC grade)
CAUTION Flammable liquid, Handle as hazardous waste and keep away from open flames.
EQUIPMENT
Surgical Clippers
Disposable and conventional cages
Disposable glass vials to contain at least 15 ml volume
Foil
Disposable laboratory apron
Mask
Hair net
Shoecovers
Goggles
Shoulder length gloves
Standard gloves
Absorbent pads
Yellow light source
Disposable spatulas
REAGENT SETUP
2500 nmol/0.2 ml acetone DMBA stock solution (100X)
Under yellow or subdued light and inside a dedicated carcinogen weighing room, measure 15 mg of DMBA into a glass vial using a disposable spatula. Add 4.68 ml acetone and mix by gentle agitation. Prior to use, dilute the 100X stock solution to 1X (25 nmol/0.2 mL acetone final concentration) by adding 150 µl of the 100X stock solution to 14.85 ml of acetone. Protect all DMBA solutions from light by wrapping vials in foil or using brown glass. The DMBA solution should be made fresh on the day of initiation. !CAUTION—DMBA is carcinogenic. Wear protective clothing (hair net, mask, disposable gown, goggles, and shoulder-length gloves layered with standard gloves) when handling solid DMBA or DMBA-containing solutions. All items that may have come into contact with DMBA as well as excess DMBA solutions should be disposed of as hazardous waste. Ideally, an isolated area in the laboratory should be designated for handling DMBA.
27.2 nmol/0.2 ml concentrated TPA stock solution
To make a stock solution of 27.2 nmol TPA/0.2 ml of acetone, dissolve 25 mg of TPA in acetone for a final volume of 298 ml. Make serial dilutions in acetone to achieve final working solutions of 1.7, 3.4, and 6.8 nmol TPA in 0.2 ml of acetone. For example, to make 120 ml of 6.8 nmol TPA/0.2 ml acetone, dilute 30 ml of the 27.2 nmol TPA/0.2 ml acetone concentrated stock in 90 ml of acetone. Subsequently, dilute 50 ml of the 6.8 nmol TPA/0.2 ml acetone stock in 50 ml of acetone to achieve a 3.4 nmol TPA/0.2 ml acetone solution. Finally, dilute 30 ml of the 3.4 nmol TPA/0.2 ml acetone solution in 30 ml of acetone to generate the 1.7 nmol TPA/0.2 ml of acetone working solution. The TPA solutions may be stored at −20 °C for at least one month.
EQUIPMENT SETUP
Disposable caging
Mice should be housed in disposable caging in a room equipped with yellow light prior to initiation with DMBA. !CAUTION--Following application of DMBA, avoid unnecessary handling of the mice for a period of two weeks. At the end of two weeks, mice may be rehoused in conventional caging and the DMBA-contaminated cages and bedding disposed as hazardous waste.
DMBA treatment hood
The application of DMBA should occur in a Class IIB2 hood. The floor of the hood should be covered with absorbent pads that are secured by laboratory or masking tape. !CAUTION--All items that may have come into contact with DMBA should be disposed as biohazardous waste or decontaminated as described below.
Pipettes for delivery of DMBA
!CAUTION—Pipettes used for the application of 0.2 mL 1X DMBA solution should not be used for any other purposes. Following application of DMBA the surfaces of the pipette should be decontaminated by wiping with ethanol. The pipette tips and tissues used for cleaning the pipettes should be disposed as biohazardous waste.
PROCEDURE
Tumor initiation TIMING ~1–2 hr for 50 mice followed by 1–2 week waiting period
-
1
Obtain a sufficient number of age-matched mice per test group.
!CAUTION—All experiments involving animals should be undertaken in compliance with national and institutional regulations.
?TROUBLESHOOTING
-
2
Shave the dorsal skin of the mice using surgical clippers. Gently restrain mice by the tail for no longer than 1–2 min while hair is removed.
!CRITICAL STEP-- Hair regrowth may interfere with uniform application of the initiating agent and the hair cycle should be synchronized as much as possible before treatment.
-
3
Randomize the mice into treatment groups.
-
4
Rehouse mice in disposable biohazard cages and relocate cages to a carcinogen treatment room equipped with a yellow light source.
-
5
Two days after shaving, the mice should be ‘initiated’. Place a freshly prepared stock of 25 nmol/0.2 ml DMBA (1X), a 20–200 microliter pipette and 300 microliter filter tips in the treatment hood under yellow light.
-
6
Place a single cage of mice in the hood along with an extra cage bottom with fresh bedding.
-
7
Gently restrain a mouse by the tail and apply 0.2 mL of 1X DMBA solution to the dorsal skin. Restrain the mouse for an additional 5 to 10 seconds to allow the acetone solution to evaporate. Release the treated mouse into the extra empty cage bottom. Repeat for the remaining mice. Control mice receive 0.2 ml acetone only.
!CAUTION— DMBA is a hazardous mutagenic compound. Avoid contact and dispose of any unused DMBA solution as hazardous waste.
-
8
Isolate the mice in disposable biohazard caging for 1–2 weeks following application of DMBA.
!CAUTION-The mice should be handled as infrequently as possible during this period to avoid personal exposure to DMBA.
Tumor promotion TIMING ~0.5–1 hr for 50 mice, twice weekly for up to 52 weeks
-
9
After two weeks have passed, rehouse the mice in conventional caging.
-
10
Begin twice weekly applications of 1.7, 3.4, and 6.8 nmol TPA in 0.2 ml of acetone. Maintain either a Monday/Thursday or Tuesday/Friday treatment schedule for the duration of the study.
-
11
Continue twice weekly application of the TPA doses in 0.2 ml of acetone for up to 52 weeks.
Data acquisition TIMING ~1 hr for 50 mice, once weekly for up to 52 weeks
-
12Beginning at approximately 6 weeks of tumor promotion with TPA, palpate the back of each mouse once-weekly to detect tumor formation. Each week record the number of tumors detected as well as the number of mice remaining in the study; document any palpable mass ≥1 mm in size. Tumor volume may also be estimated and recorded periodically104,105.
- ?TROUBLESHOOTING
-
13Monitor body weight at regular intervals to insure that test and control mice maintain approximately equal rates of weight gain.
- ?TROUBLESHOOTING
-
14
Monitor mice for the conversion of papillomas to SCCs and notate findings.
-
15
Note date and circumstance of death for any mice that die during the study. When possible, kill any mice where death appears imminent, and harvest tumor tissues for histological verification.
-
16
Kill the mice 2 weeks after the final TPA application according to Institutional Animal Care and Use Committee (IACUC) guidelines. Harvest tumors for histological verification and further study. Tumors and adjacent skin may be snap frozen, cryopreserved in optimum cutting temperature (OCT) compound or fixed in ethanol or formalin depending upon predicted experimental needs.
TIMING
See Figure 1 for protocol timeline including approximate anticipated time of appearance for papillomas and SCCs.
Steps 1–8 Tumor initiation 1–2 hr for 50 mice and 1–2 week waiting period
Steps 9–11 Tumor promotion 0.5–1 hr dosing twice weekly
Steps 12–16 Data Acquisition 1 hr tumor palpation once weekly
TROUBLESHOOTING
See Table 3 for troubleshooting advice.
Table 3.
TROUBLESHOOTING
| Step | Problem | Possible Reason | Solution |
|---|---|---|---|
| 1–16 | Wounds noted on dorsal skin | Mice are fighting | Separate the aggressor, individually house all males and aggressive females; Interpret results with caution since wounding promotes tumor development |
| 13–16 | Weight loss | Toxicity associated with chemotherapeutic or chemopreventive agent | Lower the dose of chemopreventive or chemotherapeutic agent; Interpret results with caution since negative energy balance inhibits tumor response |
| Lack of food consumption; Dietary delivery of chemotherapeutic or chemopreventive agent has made chow less palatable | Change route of delivery; Interpret results with caution since negative energy balance inhibits tumor response | ||
| Cachexia due to large tumor burden or malignant progression | Sacrifice individual mice and harvest tissue; terminate the experiment if indicated by IACUC regulations. Note: Papilloma counts may be unreliable after the appearance of SCC. | ||
| In subsequent experiments reduce the dose of DMBA and/or TPA | |||
| 12–16 | No tumors arise in positive control group | Resistant strain was used as a positive control; Dose of initiating or promoting agent too low | Use a sensitive strain, backcross transgene or gene deficiency allele to sensitive strain such as FVB. Increase the doses of DMBA/TPA. |
| Improper composition, storage, or handling of DMBA or TPA solutions | Verify solution composition; Prepare a fresh stock of DMBA for each initiation and protect from light; Store TPA solutions at −20°C. | ||
| 12–16 | Predicted effect of therapeutic or preventative agent or genetic manipulation is not detected | True negative result | Do nothing |
| False negative results may occur if the doses of DMBA/TPA overwhelm the potential preventative or therapeutic effect of treatment or genetic manipulation | Lower the dose of DMBA/TPA | ||
| Inadequate statistical power | Recalculate the necessary sample size based upon newly collected preliminary data | ||
| 12–16 | Abnormal lesions form | Transgene or inhibitor conferred unexpected gross appearance of papillomas | Harvest tumors tissues, cut sections and perform histologic investigation. |
| 12–16 | Papillomas arise but a portion regresses | Tumor regression is normal and the rate at which it occurs depends upon the strain utilized | Adjust tumor incidence and multiplicity calculations as necessary |
ANTICIPATED RESULTS
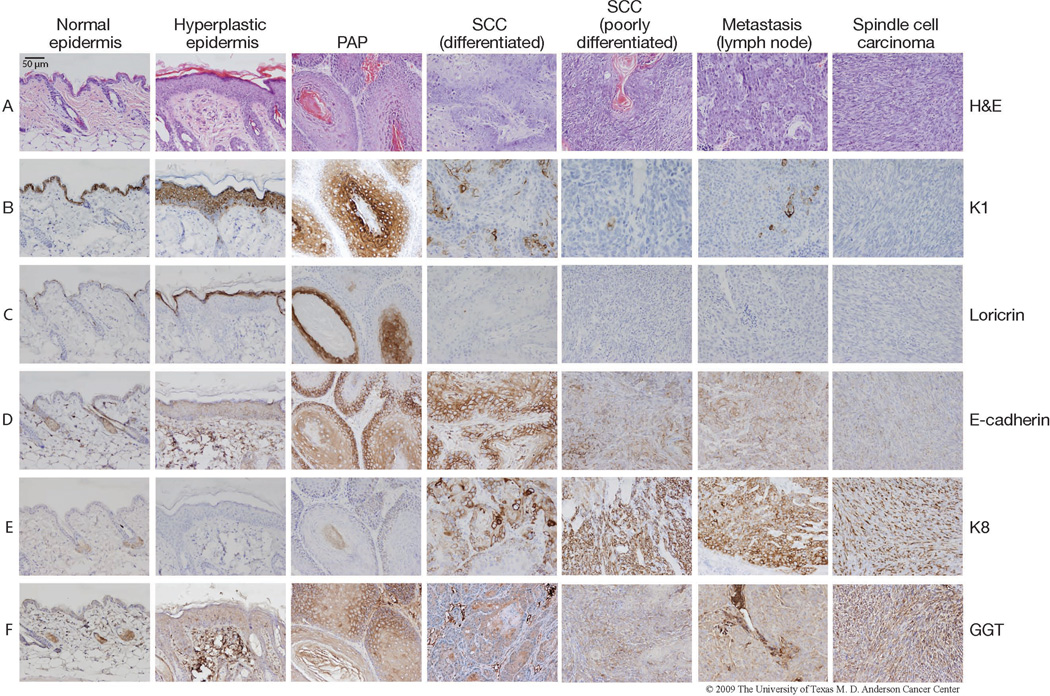
During the promotion stage, repeated TPA treatment results in sustained hyperplasia of the epidermis and, eventually, the selective clonal expansion of initiated cells into premalignant papillomas (Figs. 1 and 2). Papillomas can be expected to appear on the dorsal skin of FVB mice by ~6–8 weeks of promotion (for example data see Fig. 3). The predicted tumor incidence and tumor multiplicity for various stocks and strains of mice, including FVB, are shown in Table 2. By approximately 20 weeks of promotion, a fraction of papillomas will begin to convert to SCCs by becoming increasingly invasive and penetrating deeper into the dermis. As SCCs evolve, cellular architecture may become disorganized and cells become anaplastic, losing normal polarity as well as markers of differentiation (tumors with disorganized cells are referred to as poorly differentiated SCCs as opposed to well-differentiated SCCs (Fig. 2)). A small percentage of SCCs may convert into poorly differentiated spindle cell carcinomas that are composed of fibroblast-like carcinoma cells (Fig. 2). A number of proteins exhibit distinctive expression patterns during the promotion and progression of skin tumors in mice and can serve as biomarkers for the pathologic changes associated with the later stages of skin carcinogenesis in mice. Figure 2 shows the representative expression of several marker proteins (K1, loricrin, E-cadherin, K8 and GGT) in each sequential stage of mouse skin carcinogenesis (normal epidermis, hyperplastic epidermis, papilloma, SCC (differentiated), SCC (poorly differentiated), and spindle cell carcinoma). Staining for these markers is also shown for a regional lymph node metastasis that was harvested from the same mouse bearing the differentiated SCC in this figure.
Figure 2. Expression of several marker proteins in each sequential stage of skin carcinogenesis in mice.
Tumor tissue was harvested from FVB mice that had undergone two-stage skin carcinogenesis initiated by DMBA and promoted by TPA. The tumors as well as hyperplastic dorsal skin from between tumors and untreated ventral skin were fixed in formalin and embedded in paraffin for immunohistochemical analyses. Representative images of normal epidermis, hyperplastic epidermis, papilloma (PAP), differentiated SCC, poorly differentiated SCC, lymph node metastasis and spindle cell carcinoma are shown. The lymph node metastasis was harvested from the same mouse bearing the poorly differentiated SCC. Immunostaining using the following antibodies was performed at the Histology Core at M.D. Anderson Cancer Center Science Park-Research Division as previously described24: Loricrin (Covance, Princeton, NJ), K1 (Covance), K8 (Origene, Rockville, MD), K15 (Covance), E-cadherin (Santa Cruz), and GTT (Abcam, Cambridge, MA). All mice were handled in accordance with institutional and national regulations.
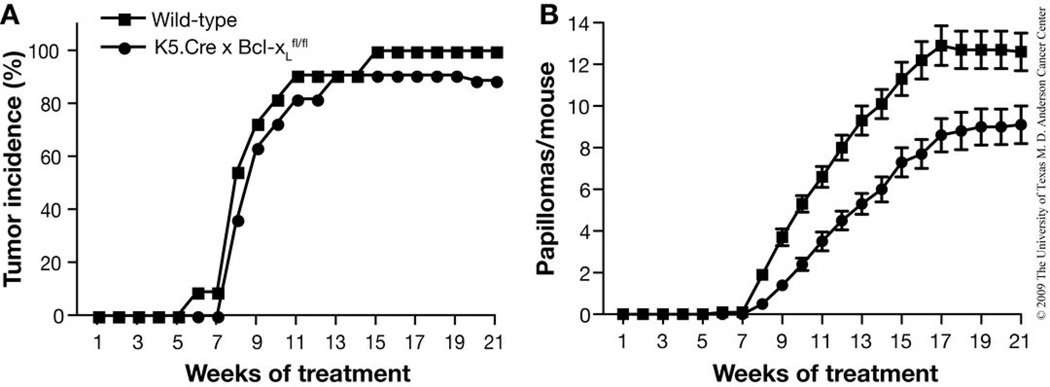
Figure 3.
Representative data from a previously-published two-stage skin carcinogenesis study in FVB mice120. This study was designed to test the effect of Bcl-xL deficiency on tumor development in the two-stage skin carcinogenesis model. BK5.Cre × Bcl-xL mice (lack Bcl-xL expression in the basal layer of the epidermis) and wild-type mice (n=11 per group) were initiated with 25 nmol DMBA and promoted twice weekly with 6.8 nmol TPA. Tumor incidence (A) and multiplicity (B) were monitored until the maximum papilloma response was achieved (21 weeks). In panel B, the average number (mean ±SEM) of papillomas per mouse is presented. Bcl-xL deficiency significantly reduced tumor multiplicity (P < 0.05 by Mann-Whitney U test). All mice were handled in accordance with institutional and national regulations.
The histologic and cytogenetic abnormalities that occur during mouse skin carcinogenesis have been shown to occur in tandem with changes in the expression patterns of select keratin genes. Keratin 14 and keratin 5 (K14 and K5, respectively) are primarily expressed in the basal cells of the proliferative compartment of the epidermis. As keratinocytes differentiate and migrate to the suprabasal layer of the epidermis, the expression of K14 and K5 decreases while the expression of keratin 1 (K1) and its partner, keratin 10 (K10), increases. Loss of K1 and K10 expression (Fig. 2b) combined with an accompanying increase in aberrantly expressed K13 (not pictured) are characteristic gene expression patterns during the progression of mouse skin papillomas to SCCs106–109. As the expression of K1 and K10 is suppressed, expression of keratin 8 (K8) is then observed in SCCs (Fig. 2e)110. Aberrant expression of K8 has been noted in SCCs, but not in papillomas, generated by the DMBA-TPA two-stage skin carcinogenesis protocol111. While K13-positive foci are primarily found in well-differentiated regions, K8-positive cells in SCCs are found primarily in anaplastic areas, suggesting that the expression of K8 can serve as a useful marker of the later stages of tumor progression in this model.
Loricrin expression is an additional marker for differentiation in mouse skin and skin tumors. Loricrin is expressed during the late terminal differentiation of keratinocytes and is the major protein of the epidermal cornified cell envelope (Fig. 2c)112. Loricrin is expressed in the granular layer of the epidermis where it accumulates in granules before it is integrated into the developing cornified envelope113,114. Whereas cornified cells and some granular cells within hyperplastic epidermis and papillomas are positive for loricrin, expression is abruptly decreased in SCCs and spindle cell carcinomas (Fig. 2c).
The expression of gamma-glutamyltransferase (GGT) and E-cadherin are additional markers of tumor progression in this model. GGT, a cell surface enzyme involved in cellular glutathione homeostasis, is present in the plasma membrane. It has been reported that GGT expression is detectable in papillomas while only a percentage of SCCs express GGT24,115. In Figure 2, some of the cells in the suprabasal layer are positive for GGT. GGT expression is widespread in papillomas but becomes more focal in SCCs (Fig. 2f). Spindle cell carcinomas show widespread positive staining for GGT (Fig. 2f). E-cadherin is a calcium-dependent cell adhesion molecule that is expressed primarily on the surface of epithelial cells. E-cadherin plays a major role in cell-cell interactions in epithelial tissues and it is well established that decreased E-cadherin function plays a critical role in the progression of SCCs51,116,117. A sequential loss of E-cadherin in mouse skin treated with both the two-stage carcinogenesis protocol and complete carcinogenesis with UV has been shown as lesions progress from dysplasia to SCCs and spindle cell carcinomas (Fig. 2d)51,118,119. In Figure 2, E-cadherin staining intensity is markedly reduced from normal skin epidermis to hyperplastic epidermis, papillomas, and differentiated SCCs. In poorly differentiated SCCs, E-cadherin staining is scattered in small isolated areas and its expression is significantly decreased in spindle cell carcinomas. Additionally, expression of markers of differentiation as well as GGT is also quite low in poorly differentiated SCCs, while K8 expression is high (Fig. 2b, 2c, 2e, 2f).
Tumor response data from a two-stage skin carcinogenesis study may be displayed in a number of ways. To reflect the longitudinal nature of the experiment, tumor incidence may be plotted as either the percentage of mice bearing any papilloma over time or as fractional tumor-free survival over time20. Either plot highlights differences in tumor latency between groups. Additionally, overall tumor burden is displayed as tumor multiplicity over time (calculated as the total number of papillomas detected in a group divided by the total number of mice in the group). As an example, please see our previously published findings presented in Figure 3120. In this study, the effect of targeted deletion of Bcl2l1 (which encodes Bcl-xL) on skin tumor development in FVB mice was analyzed120. In this case, we utilized mice with floxed alleles for Bcl-xL and targeted deletion of Bcl-xL to the basal layer of the epidermis using the bovine keratin 5 (BK5) promoter. The mice were initiated with 25 nmol DMBA and promoted twice weekly with 6.8 nmol TPA. Bcl-xL deficiency did not significantly affect tumor incidence (Fig. 3a) but resulted in significantly reduced tumor multiplicity (i.e., average number of papillomas per mouse) (Fig. 3b, P < 0.05 by Mann-Whitney U test). Similar plots may be constructed for the incidence and multiplicity of SCCs20.
Mice are unlikely to experience adverse health effects due to papilloma burden during the analysis of tumor promotion. Less than 10% of the mice are expected to die during the course of the study; therefore, papilloma incidence and multiplicity are typically calculated by dividing either the number of tumor bearing mice or total number of papillomas by the number of mice alive at each week. In contrast, mice will likely die or require sacrifice due to the presence of SCCs. In this case, SCC incidence and multiplicity are calculated cumulatively. Any SCCs that appear are carried forward, even after tumor bearing mice are sacrificed or die, and the total number of SCCs is divided by the number of mice alive at the time that the first SCC was macroscopically observed. In some instances it may be desirable to score papilloma data cumulatively; in this case the data are handled similarly to that for SCCs. In addition to tumor incidence and multiplicity, a plot of the average tumor volume per mouse at an interim time point as well as at the conclusion of the tumor study can be a useful way to communicate alteration in tumor size that occurs in the absence of an effect on tumor incidence or multiplicity. Statistical analysis of differences in tumor multiplicity should be performed using non-parametric methods since tumor burden data is non-normative20. The Mann-Whitney U test is recommended. The χ2 test is appropriate for comparing tumor incidence between groups.
VARIATIONS
Modifications of the traditional two-stage skin carcinogenesis protocol have been developed for a number of purposes. One purpose is to facilitate the study of tumor progression. For instance, it has been reported that papillomas that are at “high risk” for conversion to SCC can be generated and distinguished by gene expression profile from “low risk” papillomas121,122. In these protocols, SENCAR mice are initiated with 5 µg DMBA and then promoted once weekly with 2 µg TPA for varying amounts of time. Those papillomas that arise after only 5–10 weeks of TPA treatment are considered to be at high risk of conversion to SCC. Low risk papillomas are those papillomas that are present after 14–15 weeks of treatment with TPA.
Additionally, a “three stage” model has been suggested wherein tumor progression is accelerated by application of initiating agents to papillomas generated in the initiation-promotion protocol53,123,124. MNNG, 4-nitroquinoline-N-oxide (4-NQO), cisplatin, and urethane have all been shown to enhance malignant progression of papillomas. In this protocol, SENCAR mice are initiated with 20 µg DMBA and then promoted weekly with 2.5 µg TPA for 10 weeks. During weeks 11–40, mice are either treated topically with 250 µg 4-NQO or injected intraperitoneally with 20 mg urethane. Under these conditions, more than 60% of the SENCAR mice will develop SCC by the end of the study Alternatively, CD-1 mice may be initiated with 50 µg DMBA and then promoted weekly with 10 µg TPA for 12 weeks. At week 13, the mice are given either a single intraperitoneal injection of 100 µg cisplatin or weekly treatments with the doses of mutating agents listed above. In CD-1 mice, these treatment protocols significantly increase the conversion rate but the overall number of carcinomas/mouse is lower since fewer papillomas form during the second stage, tumor promotion.
ACKNOWLEDGEMENTS
This work was supported by NCI grants CA076520, CA37111, CA016672 and the National Institute of Environmental Health Sciences Center Grant ES007784. We wish to thank the Histology and Tissue Processing Core Facility for their technical assistance in immunohistochemical analyses, and Dr. Joyce Rundhaug for providing tumor samples. We also thank Shawna Johnson for her assistance in the preparation of this manuscript.
Footnotes
Competing Financial Interests statement:
The authors declare that they have no competing financial interests.
Author Contributions statement:
Erika Abel and John DiGiovanni were responsible for overall manuscript writing including compilation of the supporting data and procedure descriptions. Joe Angel was responsible for compiling and describing the data in Table 1. Kaoru Kiguchi was responsible for creating and describing the results of Figure 2. Numerous laboratory groups and individuals have contributed to the design and refinement of the two-stage skin carcinogenesis protocol in mice.
REFERENCES
- 1.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 2.Kemp CJ. Multistep skin cancer in mice as a model to study the evolution of cancer cells. Semin Cancer Biol. 2005;15:460–473. doi: 10.1016/j.semcancer.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Verma AK, Wheeler DL, Aziz MH, Manoharan H. Protein kinase Cepsilon and development of squamous cell carcinoma, the nonmelanoma human skin cancer. Mol Carcinog. 2006;45:381–388. doi: 10.1002/mc.20230. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein S, Hoot K, Han GW, Lu SL, Wang XJ. Distinct roles of individual Smads in skin carcinogenesis. Mol Carcinog. 2007;46:660–664. doi: 10.1002/mc.20336. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Bowden GT. Targeting Bcl-X(L) for prevention and therapy of skin cancer. Mol Carcinog. 2007;46:665–670. doi: 10.1002/mc.20330. [DOI] [PubMed] [Google Scholar]
- 6.Kim DJ, Chan KS, Sano S, DiGiovanni J. Signal transducer and activator of transcription 3 (Stat3) in epithelial carcinogenesis. Mol Carcinog. 2007;46:725–731. doi: 10.1002/mc.20342. [DOI] [PubMed] [Google Scholar]
- 7.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J Dermatol Sci. 1998;17:1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 8.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis--thirty-third G. H. A. Clowes Memorial Award Lecture. Cancer Res. 1994;54:1178–1189. [PubMed] [Google Scholar]
- 9.Slaga TJ. Cellular and molecular mechanisms involved in multistage skin carcinogenesis. In: Conti CJ, Slaga TJ, Klein-Szanto AJP, editors. Carcinogenesis: A comprehensive Survey Skin Tumors: Experimental and Clinical Aspects. Vol. 11. New York, NY: Raven Press; 1989. pp. 1–18. [PubMed] [Google Scholar]
- 10.Rundhaug JE, Fischer SM. Tumor Promoters and Models of Promotion. In: Sipes IG, McQueen CA, Gandolfi AJ, editors. Comprehensive Toxicology. Vol. 12. New York City: Elsevier Sciences Ltd; 1997. pp. 325–348. [Google Scholar]
- 11.Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets. Biochem Pharmacol. 2006;72:1506–1515. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Fujiki H, Atsumasa K, Suganuma M. Chemoprevention of Cancer. In: Bowden GT, Fischer SM, editors. Comprehensive Toxicology. Vol. 12. Oxford, UK: Pergamon; 1997. pp. 453–471. [Google Scholar]
- 13.DiGiovanni J. Modification of Multistage Skin Carcinogenesis in Mice. In: Ito N, Sugano H, editors. Modification of Tumor Development in Rodents. Vol. 33. Basel, Switzerland: Karger; 1991. pp. 192–229. [DOI] [PubMed] [Google Scholar]
- 14.Wilker E, et al. Role of PI3K/Akt signaling in insulin-like growth factor-1 (IGF-1) skin tumor promotion. Mol Carcinog. 2005;44:137–145. doi: 10.1002/mc.20132. [DOI] [PubMed] [Google Scholar]
- 15.Amornphimoltham P, Leelahavanichkul K, Molinolo A, Patel V, Gutkind JS. Inhibition of Mammalian target of rapamycin by rapamycin causes the regression of carcinogen-induced skin tumor lesions. Clin Cancer Res. 2008;14:8094–8101. doi: 10.1158/1078-0432.CCR-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol. 1998;8:516–524. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]
- 17.Kemp CJ, Donehower LA, Bradley A, Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell. 1993;74:813–822. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
- 18.Glick AB, et al. Targeted deletion of the TGF-beta 1 gene causes rapid progression to squamous cell carcinoma. Genes Dev. 1994;8:2429–2440. doi: 10.1101/gad.8.20.2429. [DOI] [PubMed] [Google Scholar]
- 19.Han G, et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115:1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto T, et al. Targeted expression of c-Src in epidermal basal cells leads to enhanced skin tumor promotion, malignant progression, and metastasis. Cancer Res. 2003;63:4819–4828. [PubMed] [Google Scholar]
- 21.Chan KS, et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rundhaug JE, Pavone A, Kim E, Fischer SM. The effect of cyclooxygenase-2 overexpression on skin carcinogenesis is context dependent. Mol Carcinog. 2007;46:981–992. doi: 10.1002/mc.20340. [DOI] [PubMed] [Google Scholar]
- 23.Segrelles C, et al. Deregulated activity of Akt in epithelial basal cells induces spontaneous tumors and heightened sensitivity to skin carcinogenesis. Cancer Res. 2007;67:10879–10888. doi: 10.1158/0008-5472.CAN-07-2564. [DOI] [PubMed] [Google Scholar]
- 24.Rundhaug JE, et al. Changes in protein expression during multistage mouse skin carcinogenesis. Mol Carcinog. 1997;20:125–136. [PubMed] [Google Scholar]
- 25.Bassi DE, Klein-Szanto AJP. Current Protocols in Pharmacology. Vol. John Wiley & Sons; 2007. Carcinogen-Induced Animal Models of Head and Neck Squamous Cell Carcinoma; pp. 14.12.11–14.12.19. [DOI] [PubMed] [Google Scholar]
- 26.Ward JM, Rehm S, Devor D, Hennings H, Wenk ML. Differential carcinogenic effects of intraperitoneal initiation with 7,12-dimethylbenz(a)anthracene or urethane and topical promotion with 12-O-tetradecanoylphorbol-13-acetate in skin and internal tissues of female SENCAR and BALB/c mice. Environ Health Perspect. 1986;68:61–68. doi: 10.1289/ehp.866861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ise K, et al. Targeted deletion of the H-ras gene decreases tumor formation in mouse skin carcinogenesis. Oncogene. 2000;19:2951–2956. doi: 10.1038/sj.onc.1203600. [DOI] [PubMed] [Google Scholar]
- 28.Pierceall WE, Kripke ML, Ananthaswamy HN. N-ras mutation in ultraviolet radiation-induced murine skin cancers. Cancer Res. 1992;52:3946–3951. [PubMed] [Google Scholar]
- 29.Rehman I, et al. Frequent codon 12 Ki-ras mutations in mouse skin tumors initiated by N-methyl-N'-nitro-N-nitrosoguanidine and promoted by mezerein. Mol Carcinog. 2000;27:298–307. [PubMed] [Google Scholar]
- 30.Nelson MA, Futscher BW, Kinsella T, Wymer J, Bowden GT. Detection of mutant Ha-ras genes in chemically initiated mouse skin epidermis before the development of benign tumors. Proc Natl Acad Sci U S A. 1992;89:6398–6402. doi: 10.1073/pnas.89.14.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balmain A, Ramsden M, Bowden GT, Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984;307:658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- 32.Brown K, Buchmann A, Balmain A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc Natl Acad Sci U S A. 1990;87:538–542. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spalding JW, Momma J, Elwell MR, Tennant RW. Chemically induced skin carcinogenesis in a transgenic mouse line (TG.AC) carrying a v-Ha-ras gene. Carcinogenesis. 1993;14:1335–1341. doi: 10.1093/carcin/14.7.1335. [DOI] [PubMed] [Google Scholar]
- 34.Morris RJ. A perspective on keratinocyte stem cells as targets for skin carcinogenesis. Differentiation. 2004;72:381–386. doi: 10.1111/j.1432-0436.2004.07208004.x. [DOI] [PubMed] [Google Scholar]
- 35.Klein-Szanto AJP. Pathology of human and experimental skin tumors. In: Conti CJ, Slaga TJ, Klein-Szanto AJP, editors. Skin Tumors: Experimental and Clinical Aspects. New York City: Raven Press; 1989. pp. 19–53. [Google Scholar]
- 36.Yuspa SH, Ben T, Hennings H, Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982;42:2344–2349. [PubMed] [Google Scholar]
- 37.Karen J, et al. 12-O-tetradecanoylphorbol-13-acetate induces clonal expansion of potentially malignant keratinocytes in a tissue model of early neoplastic progression. Cancer Res. 1999;59:474–481. [PubMed] [Google Scholar]
- 38.Hennings H, Michael D, Lichti U, Yuspa SH. Response of carcinogen-altered mouse epidermal cells to phorbol ester tumor promoters and calcium. J Invest Dermatol. 1987;88:60–65. doi: 10.1111/1523-1747.ep12465014. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson EK. Defective responses of transformed keratinocytes to terminal differentiation stimuli. Their role in epidermal tumour promotion by phorbol esters and by deep skin wounding. Br. J. Cancer. 1985;52:479–493. doi: 10.1038/bjc.1985.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodworth CD, et al. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004;25:1771–1778. doi: 10.1093/carcin/bgh170. [DOI] [PubMed] [Google Scholar]
- 41.Stern MC, Benavides F, LaCava M, Conti CJ. Genetic analyses of mouse skin tumor progression susceptibility using SENCAR inbred derived strains. Mol Carcinog. 2002;35:13–20. doi: 10.1002/mc.10067. [DOI] [PubMed] [Google Scholar]
- 42.Hennings H, et al. FVB/N mice: an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin. Carcinogenesis. 1993;14:2353–2358. doi: 10.1093/carcin/14.11.2353. [DOI] [PubMed] [Google Scholar]
- 43.Ewing MW, Conti CJ, Kruszewski FH, Slaga TJ, DiGiovanni J. Tumor progression in Sencar mouse skin as a function of initiator dose and promoter dose, duration, and type. Cancer Res. 1988;48:7048–7054. [PubMed] [Google Scholar]
- 44.DuBowski A, et al. Papillomas at high risk for malignant progression arising both early and late during two-stage carcinogenesis in SENCAR mice. Carcinogenesis. 1998;19:1141–1147. doi: 10.1093/carcin/19.6.1141. [DOI] [PubMed] [Google Scholar]
- 45.Aldaz CM, Conti CJ. The premalignant nature of mouse skin papillomas: histopathologic, cytogenetic, and biochemical evidence. Carcinog Compr Surv. 1989;11:227–242. [PubMed] [Google Scholar]
- 46.Aldaz CM, Conti CJ, Klein-Szanto AJ, Slaga TJ. Progressive dysplasia and aneuploidy are hallmarks of mouse skin papillomas: relevance to malignancy. Proc Natl Acad Sci U S A. 1987;84:2029–2032. doi: 10.1073/pnas.84.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conti CJ, Aldaz CM, O'Connell J, Klein-Szanto AJ, Slaga TJ. Aneuploidy, an early event in mouse skin tumor development. Carcinogenesis. 1986;7:1845–1848. doi: 10.1093/carcin/7.11.1845. [DOI] [PubMed] [Google Scholar]
- 48.Ruggeri B, et al. Alterations of the p53 tumor suppressor gene during mouse skin tumor progression. Cancer Res. 1991;51:6615–6621. [PubMed] [Google Scholar]
- 49.Aldaz CM, Trono D, Larcher F, Slaga TJ, Conti CJ. Sequential trisomization of chromosomes 6 and 7 in mouse skin premalignant lesions. Mol Carcinog. 1989;2:22–26. doi: 10.1002/mc.2940020104. [DOI] [PubMed] [Google Scholar]
- 50.Chan KS, et al. Forced expression of a constitutively active form of Stat3 in mouse epidermis enhances malignant progression of skin tumors induced by two-stage carcinogenesis. Oncogene. 2008;27:1087–1094. doi: 10.1038/sj.onc.1210726. [DOI] [PubMed] [Google Scholar]
- 51.Navarro P, et al. A role for the E-cadherin cell-cell adhesion molecule during tumor progression of mouse epidermal carcinogenesis. J Cell Biol. 1991;115:517–533. doi: 10.1083/jcb.115.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caulin C, Bauluz C, Gandarillas A, Cano A, Quintanilla M. Changes in keratin expression during malignant progression of transformed mouse epidermal keratinocytes. Exp Cell Res. 1993;204:11–21. doi: 10.1006/excr.1993.1003. [DOI] [PubMed] [Google Scholar]
- 53.Hennings H, et al. Malignant conversion and metastasis of mouse skin tumors: a comparison of SENCAR and CD-1 mice. Environ Health Perspect. 1986;68:69–74. doi: 10.1289/ehp.866869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boutwell RK. Some biological aspects of skin carcinogenesis. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- 55.Digiovanni J. Genetic determinants of susceptibility to mouse skin tumor promotions in inbred mice. New York: Marcel Dekker, Inc.; 1989. pp. 39–67. [Google Scholar]
- 56.Mahler KL, et al. Sequence divergence of Mus spretus and Mus musculus across a skin cancer susceptibility locus. BMC Genomics. 2008;9:626. doi: 10.1186/1471-2164-9-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mock BA, et al. Multigenic control of skin tumor susceptibility in SENCARA/Pt mice. Carcinogenesis. 1998;19:1109–1115. doi: 10.1093/carcin/19.6.1109. [DOI] [PubMed] [Google Scholar]
- 58.Nagase H, et al. Distinct genetic loci control development of benign and malignant skin tumours in mice. Nat Genet. 1995;10:424–429. doi: 10.1038/ng0895-424. [DOI] [PubMed] [Google Scholar]
- 59.Fujiwara K, Igarashi J, Irahara N, Kimura M, Nagase H. New chemically induced skin tumour susceptibility loci identified in a mouse backcross between FVB and dominant resistant PWK. BMC Genet. 2007;8:39. doi: 10.1186/1471-2156-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peissel B, et al. Use of intercross outbred mice and single nucleotide polymorphisms to map skin cancer modifier loci. Mamm Genome. 2001;12:291–294. doi: 10.1007/s003350010274. [DOI] [PubMed] [Google Scholar]
- 61.Angel JM, Caballero M, DiGiovanni J. Identification of novel genetic loci contributing to 12-O-tetradecanoylphorbol-13-acetate skin tumor promotion susceptibility in DBA/2 and C57BL/6 mice. Cancer research. 2003;63:2747–2751. [PubMed] [Google Scholar]
- 62.Angel JM, DiGiovanni J. Genetics of skin tumor promotion. Prog Exp Tumor Res. 1999;35:143–157. doi: 10.1159/000062010. [DOI] [PubMed] [Google Scholar]
- 63.de Koning JP, Wakabayashi Y, Nagase H, Mao JH, Balmain A. Convergence of congenic mapping and allele-specific alterations in tumors for the resolution of the Skts1 skin tumor susceptibility locus. Oncogene. 2007;26:4171–4178. doi: 10.1038/sj.onc.1210206. [DOI] [PubMed] [Google Scholar]
- 64.DiGiovanni J. Role of transforming growth factor-a and the epidermal growth factor receptor in multistage mouse skin carcinogenesis. In: Mukhtar H, editor. Skin Cancer: Mechanisms and Human Relevance. Boca Raton, FL: CRC Press, Inc.; 1995. pp. 181–197. [Google Scholar]
- 65.DiGiovanni J, Bhatt TS, Walker SE. C57BL/6 mice are resistant to tumor promotion by full thickness skin wounding. Carcinogenesis. 1993;14:319–321. doi: 10.1093/carcin/14.2.319. [DOI] [PubMed] [Google Scholar]
- 66.DiGiovanni J, Walker SC, Beltran L, Naito M, Eastin WC., Jr Evidence for a common genetic pathway controlling susceptibility to mouse skin tumor promotion by iverse classes of promoting agents. Cancer Res. 1991;51:1398–1405. [PubMed] [Google Scholar]
- 67.Imamoto A, et al. Comparison of 12-O-tetradecanoylphorbol-13-acetate and teleocidin for induction of epidermal hyperplasia, activation of epidermal PKC isozymes and skin tumor promotion in SENCAR and C57BL/6 mice. Carcinogenesis. 1993;14:719–724. doi: 10.1093/carcin/14.4.719. [DOI] [PubMed] [Google Scholar]
- 68.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 69.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 70.Leedham SJ, Wright NA. Expansion of a mutated clone: from stem cell to tumour. J Clin Pathol. 2008;61:164–171. doi: 10.1136/jcp.2006.044610. [DOI] [PubMed] [Google Scholar]
- 71.Wistuba II, et al. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene. 1999;18:643–650. doi: 10.1038/sj.onc.1202349. [DOI] [PubMed] [Google Scholar]
- 72.Segditsas S, et al. APC and the three-hit hypothesis. Oncogene. 2009;28:146–155. doi: 10.1038/onc.2008.361. [DOI] [PubMed] [Google Scholar]
- 73.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 74.Kangsamaksin T, Park HJ, Trempus CS, Morris RJ. A perspective on murine keratinocyte stem cells as targets of chemically induced skin cancer. Mol Carcinog. 2007;46:579–584. doi: 10.1002/mc.20355. [DOI] [PubMed] [Google Scholar]
- 75.Trempus CS, et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rundhaug JE, Fuscher SM, Bowden GT. Tumor Promoters and Models of Promotion. In: Bowden GT, Fischer SM, editors. Comprehensive Toxicology. Vol. 12. Oxford, UK: Pergamon; 1997. [Google Scholar]
- 77.Pitot HC, Dragan YP. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J. 1991;5:2280–2286. [PubMed] [Google Scholar]
- 78.Klein EA. Can prostate cancer be prevented? Nat Clin Pract Urol. 2005;2:24–31. doi: 10.1038/ncpuro0072. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, Roop DR. Genetically engineered mouse models for skin research: taking the next step. J Dermatol Sci. 2008;52:1–12. doi: 10.1016/j.jdermsci.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kleiner HE, Vulimiri SV, Starost MF, Reed MJ, DiGiovanni J. Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 2002;23:1667–1675. doi: 10.1093/carcin/23.10.1667. [DOI] [PubMed] [Google Scholar]
- 81.Gills JJ, et al. Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Lett. 2006;236:72–79. doi: 10.1016/j.canlet.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23:499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 83.Birt DF, Pinch HJ, Barnett T, Phan A, Dimitroff K. Inhibition of skin tumor promotion by restriction of fat and carbohydrate calories in SENCAR mice. Cancer Res. 1993;53:27–31. [PubMed] [Google Scholar]
- 84.Moore T, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila Pa) 2008;1:65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]
- 85.Stewart JW, et al. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis. 2005;26:1077–1084. doi: 10.1093/carcin/bgi051. [DOI] [PubMed] [Google Scholar]
- 86.Benjamin CL, Ananthaswamy HN. p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol. 2007;224:241–248. doi: 10.1016/j.taap.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 88.Kwei KA, Finch JS, Ranger-Moore J, Bowden GT. The role of Rac1 in maintaining malignant phenotype of mouse skin tumor cells. Cancer Lett. 2006;231:326–338. doi: 10.1016/j.canlet.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 89.Hirakawa S, et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Casanova ML, et al. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res. 2002;62:3402–3407. [PubMed] [Google Scholar]
- 91.Hirakawa S, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoot KE, et al. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008;118:2722–2732. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Naito M, Naito Y, DiGiovanni J. Comparison of the histological changes in the skin of DBA/2 and C57BL/6 mice following exposure to various promoting agents. Carcinogenesis. 1987;8:1807–1815. doi: 10.1093/carcin/8.12.1807. [DOI] [PubMed] [Google Scholar]
- 94.DiGiovanni J, et al. Further genetic analyses of skin tumor promoter susceptibility using inbred and recombinant inbred mice. Carcinogenesis. 1992;13:525–531. doi: 10.1093/carcin/13.4.525. [DOI] [PubMed] [Google Scholar]
- 95.Stern MC, et al. Analysis of two inbred strains of mice derived from the SENCAR stock with different susceptibility to skin tumor progression. Carcinogenesis. 1998;19:125–132. doi: 10.1093/carcin/19.1.125. [DOI] [PubMed] [Google Scholar]
- 96.DiGiovanni J, Slaga TJ, Boutwell RK. Comparison of the tumor-initiating activity of 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene in female SENCAR and CD-1 mice. Carcinogenesis. 1980;1:381–389. doi: 10.1093/carcin/1.5.381. [DOI] [PubMed] [Google Scholar]
- 97.Boutwell RK. The biochemistry of preneoplasia in mouse skin. Cancer Res. 1976;36:2631–2635. [PubMed] [Google Scholar]
- 98.Slaga TJ. Overview of tumor promotion in animals. Environ Health Perspect. 1983;50:3–14. doi: 10.1289/ehp.83503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slaga T, Nesnow S. SENCAR mouse skin tumorigenesis. In: HA Milman EW, editor. Handbook of Carcinogen Testing. Park Ridge: Noyes Publication; 1985. pp. 230–50. [Google Scholar]
- 100.Markel P, et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 101.Haseman JK. Statistical issues in the design, analysis and interpretation of animal carcinogenicity studies. Environ Health Perspect. 1984;58:385–392. doi: 10.1289/ehp.8458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47:5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- 103.Nutrient Requirements of Laboratory Animals. Washington, D.C: National Academy Press; 1995. Subcommittee on Laboratory Animal Nutrition, C.o.A.N., Board on Agriculture, National Research Council. [Google Scholar]
- 104.Virador VM, et al. The human promyelocytic leukemia protein is a tumor suppressor for murine skin carcinogenesis. Mol Carcinog. 2008 doi: 10.1002/mc.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santos M, et al. Susceptibility of pRb-deficient epidermis to chemical skin carcinogenesis is dependent on the p107 allele dosage. Mol Carcinog. 2008;47:815–821. doi: 10.1002/mc.20426. [DOI] [PubMed] [Google Scholar]
- 106.Roop DR, Krieg TM, Mehrel T, Cheng CK, Yuspa SH. Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res. 1988;48:3245–3252. [PubMed] [Google Scholar]
- 107.Nischt R, et al. Aberrant expression during two-stage mouse skin carcinogenesis of a type I 47-kDa keratin, K13, normally associated with terminal differentiation of internal stratified epithelia. Mol Carcinog. 1988;1:96–108. doi: 10.1002/mc.2940010205. [DOI] [PubMed] [Google Scholar]
- 108.Gimenez-Conti I, et al. Early expression of type I K13 keratin in the progression of mouse skin papillomas. Carcinogenesis. 1990;11:1995–1999. doi: 10.1093/carcin/11.11.1995. [DOI] [PubMed] [Google Scholar]
- 109.Aldaz CM, et al. Sequential development of aneuploidy, keratin modifications, and gamma-glutamyltransferase expression in mouse skin papillomas. Cancer Res. 1988;48:3253–3257. [PubMed] [Google Scholar]
- 110.Diaz-Guerra M, et al. Expression of simple epithelial cytokeratins in mouse epidermal keratinocytes harboring Harvey ras gene alterations. Cancer Res. 1992;52:680–687. [PubMed] [Google Scholar]
- 111.Larcher F, et al. Aberrant expression of the simple epithelial type II keratin 8 by mouse skin carcinomas but not papillomas. Mol Carcinog. 1992;6:112–121. doi: 10.1002/mc.2940060206. [DOI] [PubMed] [Google Scholar]
- 112.Mehrel T, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 113.Steven AC, Bisher ME, Roop DR, Steinert PM. Biosynthetic pathways of filaggrin and loricrin--two major proteins expressed by terminally differentiated epidermal keratinocytes. J Struct Biol. 1990;104:150–162. doi: 10.1016/1047-8477(90)90071-j. [DOI] [PubMed] [Google Scholar]
- 114.Bickenbach JR, Greer JM, Bundman DS, Rothnagel JA, Roop DR. Loricrin expression is coordinated with other epidermal proteins and the appearance of lipid lamellar granules in development. J Invest Dermatol. 1995;104:405–410. doi: 10.1111/1523-1747.ep12665896. [DOI] [PubMed] [Google Scholar]
- 115.Chiba M, Maley MA, Klein-Szanto AJ. Sequential study of gamma-glutamyltransferase during complete and two-stage mouse skin carcinogenesis. Cancer Res. 1986;46:259–263. [PubMed] [Google Scholar]
- 116.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 117.Margulis A, et al. Loss of intercellular adhesion activates a transition from low- to high-grade human squamous cell carcinoma. Int J Cancer. 2006;118:821–831. doi: 10.1002/ijc.21409. [DOI] [PubMed] [Google Scholar]
- 118.Brouxhon S, et al. Sequential down-regulation of E-cadherin with squamous cell carcinoma progression: loss of E-cadherin via a prostaglandin E2-EP2 dependent posttranslational mechanism. Cancer Res. 2007;67:7654–7664. doi: 10.1158/0008-5472.CAN-06-4415. [DOI] [PubMed] [Google Scholar]
- 119.Holden PR, McGuire B, Stoler A, Balmain A, Pitts JD. Changes in gap junctional intercellular communication in mouse skin carcinogenesis. Carcinogenesis. 1997;18:15–21. doi: 10.1093/carcin/18.1.15. [DOI] [PubMed] [Google Scholar]
- 120.Kim DJ, et al. Targeted disruption of Bcl-x(L) in mouse keratinocytes inhibits both UVB-and chemically induced skin carcinogenesis. Mol Carcinog. 2009 doi: 10.1002/mc.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darwiche N, et al. Expression profile of skin papillomas with high cancer risk displays a unique genetic signature that clusters with squamous cell carcinomas and predicts risk for malignant conversion. Oncogene. 2007;26:6885–6895. doi: 10.1038/sj.onc.1210491. [DOI] [PubMed] [Google Scholar]
- 122.Hennings H, Shores R, Mitchell P, Spangler EF, Yuspa SH. Induction of papillomas with a high probability of conversion to malignancy. Carcinogenesis. 1985;6:1607–1610. doi: 10.1093/carcin/6.11.1607. [DOI] [PubMed] [Google Scholar]
- 123.Hennings H, et al. Malignant conversion of mouse skin tumours is increased by tumour initiators and unaffected by tumour promoters. Nature. 1983;304:67–69. doi: 10.1038/304067a0. [DOI] [PubMed] [Google Scholar]
- 124.Hennings H, et al. Enhanced malignant conversion of benign mouse skin tumors by cisplatin. J Natl Cancer Inst. 1990;82:836–840. doi: 10.1093/jnci/82.10.836. [DOI] [PubMed] [Google Scholar]
- 125.DiGiovanni J, Kruszewski FH, Chenicek KJ. Modulation of chrysarobin skin tumor promotion. Carcinogenesis. 1988;9:1445–1450. doi: 10.1093/carcin/9.8.1445. [DOI] [PubMed] [Google Scholar]
- 126.Slaga TJ. SENCAR mouse skin tumorigenesis model versus other strains and stocks of mice. Environ Health Perspect. 1986;68:27–32. doi: 10.1289/ehp.866827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reiners JJ, Jr, Singh KP. Susceptibility of 129/SvEv mice in two-stage carcinogenesis protocols to 12-O-tetradecanoylphorbol-13-acetate promotion. Carcinogenesis. 1997;18:593–597. doi: 10.1093/carcin/18.3.593. [DOI] [PubMed] [Google Scholar]
- 128.Naito M, DiGiovanni J. Genetic background and development of skin tumors. Carcinog Compr Surv. 1989;11:187–212. [PubMed] [Google Scholar]
- 129.Naito M, Chenicek KJ, Naito Y, DiGiovanni J. Susceptibility to phorbol ester skin tumor promotion in C57BL/6 × DBA/2) F1 mice is inherited as an incomplete dominant trait: evidence for multi-locus involvement. Carcinogenesis. 1988;9:639–645. doi: 10.1093/carcin/9.4.639. [DOI] [PubMed] [Google Scholar]
- 130.Sundberg JP, Sundberg BA, Beamer WG. Comparison of chemical carcinogen skin tumor induction efficacy in inbred, mutant, and hybrid strains of mice: morphologic variations of induced tumors and absence of a papillomavirus cocarcinogen. Mol Carcinog. 1997;20:19–32. doi: 10.1002/(sici)1098-2744(199709)20:1<19::aid-mc4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 131.Bol DK, et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62:2516–2521. [PubMed] [Google Scholar]
- 132.Moore T, et al. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res. 2008;68:3680–3688. doi: 10.1158/0008-5472.CAN-07-6271. [DOI] [PubMed] [Google Scholar]
- 133.Abel E, DiGiovanni J. Environmental Carcinogenesis. In: Mendelsohn J, Howley PM, Israel M, Gray JW, Thompson CB, editors. The Molecular Basis of Cancer. Philadelphia: Elsevier; 2008. pp. 91–113. [Google Scholar]