Abstract
CD4+ T-cell dysfunction highlighted by defects within the intracellular signaling cascade and cell cycle has long been characterized as a direct and/or indirect consequence of human immunodeficiency virus (HIV) infection in humans and simian immunodeficiency virus (SIV) infection in rhesus macaques (RM). Dysregulation of the M phase of the cell cycle is a well-documented effect of HIV or SIV infection both in vivo and in vitro. In this study the effect of SIV infection on the modulation of two important regulators of the M phase—polo-like kinases Plk3 and Plk1—was investigated. We have previously shown that Plk3 is markedly downregulated in CD4+ T cells from SIV-infected disease-susceptible RM but not SIV-infected disease-resistant sooty mangabeys (SM), denoting an association of downregulation with disease progression. Here we show that, in addition to the downregulation, Plk3 exhibits aberrant activation patterns in the CD4+ T cells from SIV-infected RM following T-cell receptor stimulation. Interestingly, in vitro SIV infection of CD4+ T cells leads to the upregulation, rather than downregulation, of Plk3, suggesting that different mechanisms operate in vitro and in vivo. In addition, CD4+ T cells from RM with high viral loads exhibited consistent and significant upregulation of Plk1, concurrent with an aberrant activation-induced Plk1 response, suggesting complex mechanisms of SIV-induced M-phase abnormalities in vivo. Altogether this study presents a novel mechanism underlying M-phase defects observed in CD4+ T cells from HIV or SIV-infected disease-susceptible humans and RM which may contribute to aberrant T-cell responses and disease pathogenesis.
It is now generally accepted that it is not only the physical depletion of the CD4+ T cells but also a dysfunction of both infected and uninfected CD4+ T cells that contributes to disease progression in human immunodeficiency virus (HIV)-infected humans and simian immunodeficiency virus (SIV)-infected nonhuman primates (16, 39, 45). Both SIV and HIV infection exhibits profound effects on CD4+ T-cell signaling that affect CD4+ T-cell function, leading to insufficient CD4+ T-helper-cell function and gradually generalized unresponsiveness of the immune system (4, 43, 47). The fact that highly active antiretroviral therapy is not capable of completely eradicating the virus, accompanied by a failure to achieve full immune reconstitution (20), clearly indicates the importance of a search for new therapeutic approaches which would target these dysregulatory viral effects (33). It has been previously shown that both HIV and SIV are capable of inducing changes in cellular signaling and the cell cycle. One of the important effects that both HIV and SIV infection was shown to have on cell cycle regulation is the ability of Vpr to cause cell cycle arrest of the infected cells at the G2/M stage in vitro (15, 40). This cell cycle arrest is potentially mediated by the cascade initiated by the interaction of Vpr with the protein hVIP (32) and characterized by accumulation of phosphorylated-inactive Cdc2 (21, 24, 41). The extended G2/M block then allows for an increased production and extended release of the virus from the infected cell (19). Interestingly, however, CD4+ T cells from HIV-infected patients ex vivo showed increased and prolonged activation of Cdc2 compared to that of uninfected controls (7, 39). Activation of Cdc2 is an essential step for the progression of the cell through the G2/M phase (reviewed in reference 28) that is accomplished by the dephosphorylation of Cdc2 by protein phosphatase Cdc25C at the late S/G2 phase. Interestingly, inhibition of Cdc25C-mediated activation of Cdc2 was shown to be one of the mechanisms underlying the Vpr-mediated G2/M arrest (1, 13). Cdc25C in turn is phosphorylated-activated by polo-like kinase 1 (Plk1), leading to G2/M progression (42). Further phosphorylation of Cdc25C by polo-related kinase (Prk)/fibroblast growth factor-inducible kinase (Fnk)/polo-like kinase 3 (Plk3) during the M phase leads to the nuclear export of Cdc25C that in turn leads to the phosphorylation-inactivation of Cdc2 by negative factors, which is necessary for the cell to exit from the M phase (12, 36, 38). Plk1 and Plk3 belong to the family of polo-like kinases, which have been previously shown to play an important role in the regulation of the mitotic phase of the cell cycle in a number of cell lineages (18, 35), and the expression and phosphorylation of Plk1 and Plk3 periodically oscillate during the cell cycle (9, 22). In contrast, data from a study conducted on monocytes seem to suggest that in these cells the level of Plk3 remains constant throughout the cell cycle and that it is cell adhesion that induces Plk3 (23).
During the course of the studies aimed at characterization of the potential targets of lentivirus-mediated CD4+ T-cell dysfunction, we have utilized SIV-infected disease-susceptible rhesus macaques (RM) and naturally SIV-infected disease-resistant sooty mangabeys (SM) with the rationale that we can thus distinguish between disease-associated and nonassociated biological events (3-5). It is important to note that disease susceptibility and resistance in RM and SM, respectively, are not associated with differences in viral loads (8). We have previously identified several signaling molecules involved in both T-cell receptor (TCR) signaling cascades and cell cycle regulatory events that showed clear dysregulation in symptomatically SIV-infected RM but not in the asymptomatically infected SM (5). The most marked downregulation was noted in the levels of Plk3 in pathogenic SIV infection of RM but not in apathogenic SIV infection of SM. We have shown that CD4+ T cells from disease-susceptible SIV-infected RM harbor ∼10- to 100-fold-lower levels of Plk3 mRNA than do CD4+ T cells from SIV-infected disease-resistant SM or those from uninfected RM or SM controls. An interest in the potential role of this kinase in the pathogenesis of disease was further supported by the finding that both HIV and SIV infection leads to a profound dysregulation of the mitotic phase characterized by the dysregulation of Cdc2 (7, 21, 39, 41), which is a downstream target of both Plk3 and Plk1 (36, 42). In this study we show that Plk3 is indeed downregulated at the protein level in CD4+ T cells from pathogenically infected RM and that this downregulation is concurrent with the dysregulation of the Plk3-Cdc25C-Cdc2 pathway. Furthermore we show that Plk3 is most likely involved in CD4+ T-cell cycle regulation and that Plk1 is also dysregulated in pathogenic, but not apathogenic, SIV infection. These data suggest that dysregulation of Plk1 and Plk3 is at least one of the mechanisms that contribute to M-phase aberrations observed in CD4+ T cells in both HIV and SIV infection. Additional studies may yield important insights into the pathogenesis of lentivirus-induced cell cycle dysregulation and the identification of potential targets for future therapies.
MATERIALS AND METHODS
Cells.
The peripheral blood samples were obtained from healthy adult RM (Macaca mulatta), SIVmac251-infected RM later than 6 weeks postinfection, and healthy adult SIV-seronegative and -seropositive SM (Cercocebus atys) housed at the Yerkes Regional Primate Research Center of Emory University. All experimental groups consisted of at least three animals, and n, where appropriate, denotes the number of animals tested in each group. Viral loads were determined using quantitative competitive reverse transcription-PCR as previously described (34). All animals were maintained according to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, and the U.S. Department of Health and Human Services guidelines Guide for the Care and Use of Laboratory Animals. CD4+ T cells were isolated from fresh or cryopreserved peripheral blood mononuclear cells with Dynabeads M450 CD4 (Dynal, Lake Success, N.Y.). The purity of the cell population was always >99.0% as determined by fluorescence-activated cell sorting (FACS) analysis.
Cell culture.
Primary CD4+ T cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, gentamicin (Gibco, Carlsbad, Calif.), and 10 U of interleukin 2/ml. The HSRM1 transformed cell line was established by culturing primary CD4+ T cells with herpesvirus saimiri as previously described (2). The transformed cell line was expanded in medium prepared as described above supplemented with 50 U of interleukin 2/ml. Where appropriate, cells were incubated in medium alone (nonstimulated cells) or in medium containing anti-CD3 alone (clone FN18; Biosource International, Camarillo, Calif.) or anti-CD3-anti-CD28 (Becton Dickinson, San Jose, Calif.) antibody-conjugated immunobeads prepared as described elsewhere (6). In some experiments cells were synchronized using 100 ng of nocodazole/ml or 0.25 ng of aphidicolin/ml (both from Calbiochem, San Diego, Calif.) for 12 h. HOS-CD4-CCR5 cells were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and gentamicin (all from Life Technologies). In the in vitro SIV infection experiments purified CD4+ T cells were infected with SIVmac251 (0.1 50% tissue culture infective dose/cell) or mock infected and cultured for 8 days, and the infection-virus replication was verified using a p27 enzyme-linked immunosorbent assay (Coulter, Miami, Fla.).
Western blotting.
Cell lysates (30 μg of total cellular protein/sample) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to an Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, Calif.), and the membranes were blocked for 1 h with Tris-buffered saline-Tween 20 containing 5% milk. The membranes were probed with either anti-Fnk monoclonal antibody (MAb) (clone 53; BD Transduction Laboratories, San Diego, Calif.), anti-Cdc25C MAb (clone C2-2; BD PharMingen, San Diego, Calif.), anti-phospho-Cdc2 (Tyr15) rabbit polyclonal antibody (Cell Signaling Technology, Beverly, Mass.), anti-Plk1 (Upstate Biotechnology, Lake Placid, N.Y.), or β-actin MAb (clone AC-15; Sigma, St. Louis, Mo.). Following the addition of goat anti-mouse or goat anti-rabbit immunoglobulin-alkaline phosphatase conjugate (Biosource), the blots were developed using Lumi-Phos WB (Pierce).
Cell cycle gene expression array analysis.
RNA (1 μg/sample) was isolated from cells by using the RNAid reagent (Ambion, Austin, Tex.). The gene expression array analysis was performed using the nonradioactive cell cycle GEArray Q series kit (SuperArray Inc., Bethesda, Md.) according to the manufacturer's instructions. CD4+ T cells from four SIV-negative RM and two independent preparations of HSRM1 cells were cultured in medium or stimulated with anti-CD3 antibody-coated beads for 16 h. The level of gene transcription in each sample was quantitated by densitometry and normalized to the expression of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. Mean values for the primary CD4+ T cells and HSRM1 cell line were derived (standard deviations [SD] for each set were always <50% of the mean). Data are expressed as fold difference between gene expression observed in primary CD4+ T cells and that in the HSRM1 cell line.
Real-time PCR quantification.
cDNA samples were subjected to real-time PCR in an iCycler (Bio-Rad) and SYBR Green fluorescence quantification (PE Applied Biosystems, Foster City, Calif.) utilizing primers for Plk1 (5′-AGGCAAGAGGAGGCTGAGGA-3′ and 5′-GCACCCCCACGCTGTTATC-3′) or Plk3 (5′-TCACTGGGCTGTGTCATGTA-3′ and 5′-GTGAACCTGCTTGATGCAG-3′). Parameters of the cycle were 95°C for 15 min and 60°C for 1 min. As a control, an amplification of the GAPDH fragment was performed using the primers 5′-ACCACCATGGAGAAGGCTGG-3′ and 5′-CAGTTGGTGGTGCAGGAGGC-3′. The target cDNA quantitation in different samples was then performed according to the directions of the manufacturer (SYBR Green kit; PE Applied Biosystems) by first normalizing the threshold cycle number of the target gene to that of GAPDH. The copy numbers of the target gene were then expressed relative to the calibrator sample. Each target sequence and the GAPDH control were quantitated from two independent cDNA preparations from each sample and/or animal, and the resulting relative quantitation is expressed as an average of two measurements.
Statistical analysis.
The data were analyzed using the t test, and the differences marked by an asterisk represent P values of <0.001. Error bars represent SD for averages obtained from samples from different animals.
RESULTS
Plk3 is downregulated in CD4+ T cells in pathogenic SIV infection.
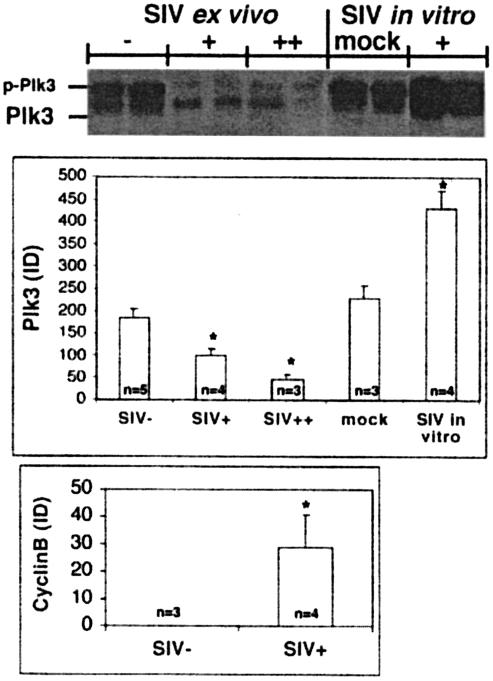
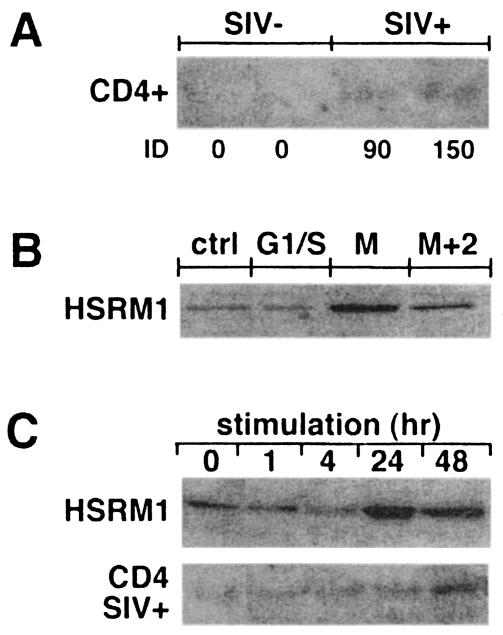
In our previous studies we found a ∼10- to 100-fold downregulation of Plk3 mRNA in CD4+ T cells obtained from disease-susceptible SIV-infected RM compared to uninfected controls. However, there was no difference in Plk3 levels between CD4+ T cells from the disease-resistant SIV-infected SM and those from uninfected SM (5). To further define the potential difference in Plk3 expression in RM, we performed Western blot analysis of lysates from CD4+ T cells from both SIV-naïve and -infected RM (Fig. 1). As can be seen, the levels of expression of Plk3 are indeed substantially lower in CD4+ T cells isolated from the peripheral blood mononuclear cells of SIV-infected RM with both low (+; <106 copies/ml) and high (++; >107 copies/ml) values compared to the noninfected controls. These cells also—similarly to the CD4+ T cells from HIV-infected patients—expressed higher levels of cyclin B, suggesting a dysregulation of the M phase in these cells.
FIG. 1.
Effect of in vivo and in vitro SIV infection on expression of Plk3. Lysates from CD4+ T cells obtained from SIV-negative (SIV−) or SIV-positive (SIV+) RM with viral loads of <106 (+) or >107 (++) copies/ml and from in vitro mock-infected (mock) and SIVmac251-infected CD4+ T cells (+) were assayed for the expression of Plk3 by Western blotting. p-Plk3 denotes a slower-migrating species of Plk3, presumably the phosphorylated form. Representative data from two animals from each group (consisting of at least three animals) are shown. Results of Western blot analysis of Plk3 and cyclin B expression were quantified by densitometry, normalized to the levels of β-actin, and expressed as average integrated density (ID). Error bars represent SD of values obtained from each experimental group containing samples from n animals, and an asterisk denotes a statistically significant difference (P < 0.001) from the SIV-negative samples.
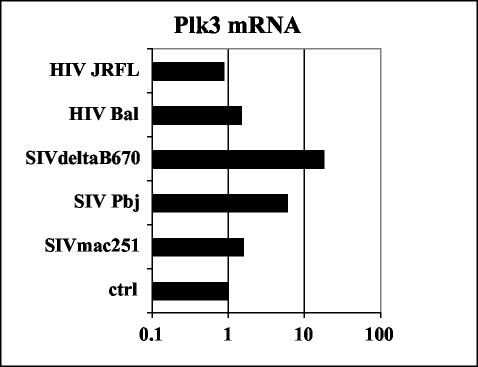
It was of interest to establish whether the downregulation of Plk3 expression was due to the direct effect of SIV infection or due rather to the indirect effect of the infection on the CD4+ T-cell compartment. As can be seen in Fig. 1 SIVmac251 infection of SIV-naïve CD4+ T cells in vitro led to a significant increase of Plk3 expression 10 days postinfection. Interestingly, in the cells from SIV-naïve animals and in in vitro-infected cells there was a prominent band representing slower-migrating species of Plk3 (p-Plk3, presumably corresponding to the phosphorylated form of Plk3). This species was present also in the CD4+ T cells from SIV-infected RM ex vivo; however, it constituted only a minor fraction of Plk3-specific signal. A similar upregulatory effect of in vitro SIV infection on Plk3 expression was observed when HOS-CD4-CCR5 cells were infected with various HIV and SIV isolates and levels of Plk3 were assessed by real-time PCR (Fig. 2). Interestingly, the magnitude of upregulation varied with the virus isolate used, ranging from no or only a minimal effect of tested HIV isolates or SIVmac251 to an ∼10-fold increase observed with SIVsmΔB670 or highly pathogenic SIVsmPbj14.
FIG. 2.
In vitro SIV-HIV infection upregulates Plk3 in HOS-CD4-CCR5 cells. HOS-CD4-CCR5 cells were infected with indicated HIV and SIV isolates, and the transcription levels of Plk3 at day 10 postinfection were assessed by real-time PCR, normalized to GAPDH, and expressed as copy numbers relative to the uninfected sample (ctrl). Average values of two independent experiments are shown.
These data therefore indicate that the downregulatory effect of in vivo SIV infection on Plk3 expression is not a direct effect of the virus on Plk3 transcription-translation but rather an indirect effect of infection on the regulation and homeostasis within the CD4+ T-cell compartment.
Plk3 levels reflect the cell cycle progression of CD4+ T cells.
To further characterize the role of Plk3 in the development of CD4+ T-cell dysfunction characteristic of AIDS, it was necessary to establish what role Plk3 plays in CD4+ T cells. It has been previously reported that this kinase is associated with the regulation of proliferation and cell cycle progression in some cell lineages, e.g., fibroblasts (9, 18, 22, 35). However, in other cell lineages, such as macrophages or neural cells, Plk3 was shown to be associated with adhesion or synaptic plasticity, respectively (23, 26).
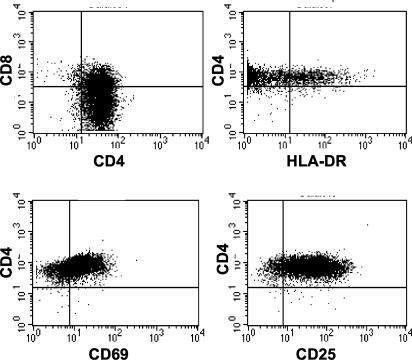
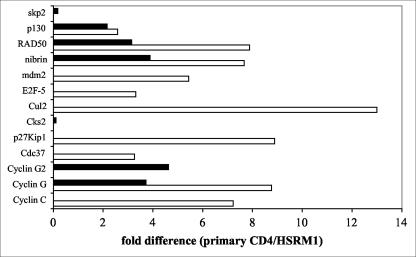
To gain further insight into the role of Plk3 in CD4+ T cells and circumvent the necessity for large numbers of primary T cells necessary for extended cell cycle studies, we have established a herpesvirus saimiri-transformed CD4+ T-cell line from RM CD4+ T cells termed HSRM1. When characterized by FACS (Fig. 3), the cells were found to be CD3 and CD4 positive, with approximately one-third of cells being CD4-CD8 double positive. The cells do not express B-cell marker CD20 and are CD16 (NK marker) and CD45RA (memory cells; there is no reagent for nonhuman primate CD45RO staining) negative. Cells were positive for the activation markers CD69 (80%) and CD25 (93%), and ∼10% of the cells were also positive for HLA-DR. Prior to use of the HSRM1 cell line it was important to establish whether these cells are comparable to primary CD4+ T cells in the parameters of cell activation-cell cycle. Therefore, a comparative protein kinase differential display analysis of the HSRM1 cells and primary CD4+ T cells was performed (5). Analysis of both nonstimulated and TCR-stimulated cells did not show any major differences in protein kinase patterns between primary cells and this transformed CD4+ T-cell line (data not shown). Furthermore, to characterize the cell cycle parameters of the transformed cell lineage, cell cycle-specific gene expression array analysis was performed (Fig. 4). Densitometric analysis of the data obtained from both the nonstimulated and anti-CD3-stimulated cells showed differences in the levels of transcription in only a few out of the 96 cell cycle-related factors included in the array. Interestingly, for all but two factors (Cks2 and Skp2), higher levels were always detected in primary CD4+ T cells. Most notably there was a ∼10-fold-higher expression of cullin in the nonstimulated primary cells. There was also fivefold-higher expression of cyclin C, cyclin G, p27Kip1, and RAD50 specific signal in resting primary cells, but this difference was unremarkable following TCR stimulation. There was also an approximately four- to fivefold-higher expression of cyclin G2 in TCR-stimulated primary cells. Overall, this analysis found the HSRM1 cell line to exhibit parameters similar to those of the parental primary CD4 T cells and established the validity for its use in further assays.
FIG. 3.
Phenotypic characterization of HSRM1 cell line. The herpesvirus saimiri-transformed CD4+ T-cell line HSRM1 was characterized for the expression of indicated surface markers by FACS.
FIG. 4.
Cell cycle-related gene expression analysis of primary CD4+ T cells and the HSRM1 cell line. Primary CD4+ T cells from four SIV-negative RM and two independent preparations of HSRM1 cells were cultured in medium (nonstimulated; white bars) or stimulated with anti-CD3 antibody-coated beads for 16 h (black bars), and transcription of cell cycle-related factors was determined using the Cell Cycle GEArray Q series kit (SuperArray). The levels of gene transcription in each sample were normalized to the expression of the GAPDH gene, and mean values for the primary CD4+ T cells and HSRM1 cell line were derived. Data are expressed as fold difference of gene expression between the primary CD4+ T cells and the HSRM1 cell line, and only those genes showing more than a threefold difference in the expression (out of 96 genes tested) are shown.
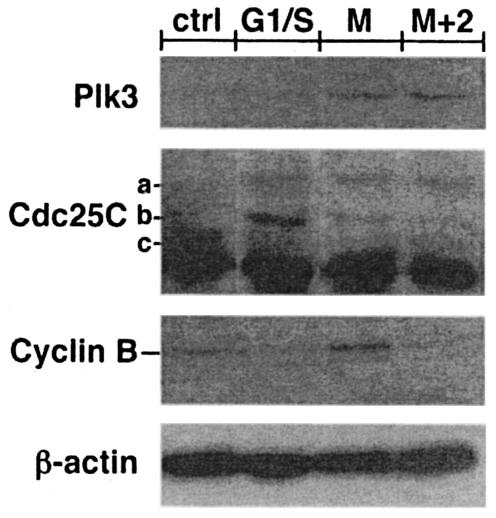
To investigate the role of Plk3 in CD4+ T cells, it was important to establish the correlation of Plk3 expression with the cell cycle progression. Therefore, the HSRM1 cell line was synchronized with aphidicolin (G1/S) or nocodazole (G2/M blocker) and the levels of Plk3 were measured by Western blotting after the release of the block (Fig. 5). While the aphidicolin treatment led to Plk3 levels indistinguishable from those of the nonsynchronized cells, there was a clear increase in the Plk3 levels in the nocodazole-synchronized cells. These levels remained slightly increased 2 h following the release of the block as cells were progressing through mitosis. The immediate downstream target of Plk3 is Cdc25C (36). Cdc25C analysis in the synchronized cells showed that nocodazole synchronization led to the disappearance of the nonphosphorylated-inactive form (band c, Fig. 5) and an increase in the hyperphosporylated-active form (band a) of Cdc25C (27). With elapsed time following block release, the hyperphosphorylated form started to disappear concurrently with an increase in the phosphorylated-inactive form (band b)—presumably as an effect of Plk3-mediated phosphorylation on Ser216. As expected the proportion of this form further increased in G1/S-blocked cells. Furthermore the levels of Plk3 and the phosphorylation status of Cdc25C correlated with mitotic cyclin B levels, and together these data suggest that Plk3 expression is probably associated with the regulation of cell cycle progression.
FIG. 5.
Expression of Plk3 correlates with the cell cycle progression in CD4+ T cells. Lysates from the HSRM1 cell line were assayed for the expression of Plk3, Cdc25C, and cyclin B at various stages of the cell cycle. Cells were either nonsynchronized (ctrl), synchronized at G1/S phase with aphidicolin (G1/S) or at G2/M phase with nocodazole (M), or assayed 2 h after the release of the nocodazole block (M+2). Cdc25C analysis shows the nonphosphorylated-inactive form (c), the single-phosphorylated-inactive form (b), and the double-phosphorylated-active form (a). β-Actin expression was used as a control for the equivalent loading.
Plk3 exhibits aberrant TCR activation response in CD4+ T cells from SIV-infected RM.
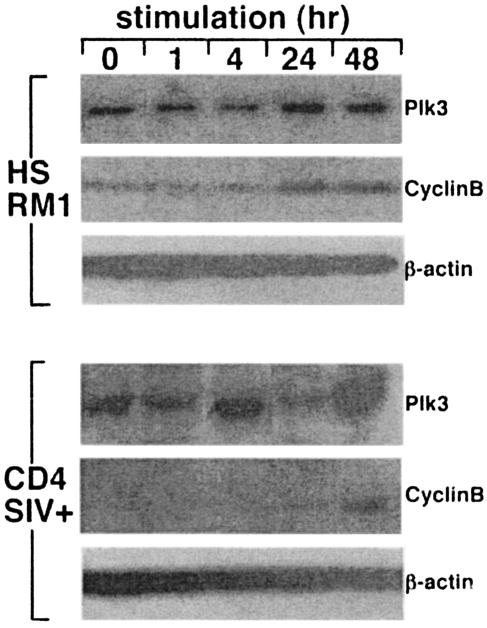
Since cell cycle progression of CD4+ T cells is a consequence of cell activation, it was important to determine whether T-cell activation has any detectable effect on Plk3 levels. The HSRM1 cells were activated by anti-CD3-CD28 stimulation for various times, and Plk3 levels were measured by Western blotting. As can be seen in Fig. 6, stimulation of these cells led to the gradual increase of Plk3 detectable as early as 4 h with a peak at 24 h and subsequent decline to baseline levels at 48 h. These levels presumably reflected progression through the cell cycle since they were followed by a gradual increase of mitotic cyclin B levels peaking at 24 h, suggesting the passage of the cells through the M phase. Similar data (∼24-h cyclic increase) were obtained when primary CD4+ T cells from uninfected RM were stimulated as described above (data not shown). This activation-induced cycle peaking at ∼24 h was similar to the activation-induced cycle previously reported in CD4+ T cells from HIV-negative human volunteers (7). Interestingly, however, similar stimulation of CD4+ T cells from SIV-infected RM led to a rapid increase of Plk3 peaking at 4 h and to an aberrant cyclic pattern thereafter, e.g., a decrease to baseline levels at 24 h followed by another increase at 48 h. At the same time these rapid cyclic changes in Plk3 were not followed by an increase in cyclin B, levels of which peaked at 48 h, indicating an aberrant cell cycle. These data suggested that the Plk3 levels are indeed associated with activation in CD4+ T cells and that the SIV infection—besides its effect on the baseline levels of Plk3—leads to the aberrant kinetics of activation-induced Plk3 response and cell cycle.
FIG. 6.
Plk3 expression in CD4+ T cells from SIV-infected RM exhibits aberrant kinetics after stimulation. HSRM1 cells and CD4+ T cells from SIV-infected RM were stimulated with anti-CD3-anti-CD28 antibody-coated beads for the indicated time intervals and assayed for the expression of Plk3 and cyclin B; β-actin expression was used as a control for the equivalent loading. For primary CD4+ T cells representative data from a group of three animals are shown.
Plk1 is upregulated by high levels of SIV infection.
Plk3 has been shown to phosphorylate Cdc25C on Ser216, leading to its inactivation and nuclear export. However, Plk1, another polo-like kinase, has been shown to activate Cdc25C by phosphorylation on Thr198, leading to its nuclear import and initiation of the M phase (48). At the same time, Plk1 also has been shown to phosphorylate cyclin B, leading similarly to its activation and nuclear import (49).
To further investigate other potential mechanisms by which chronic SIV infection can affect the Cdc25C pathway-mediated regulation of the M phase, a real-time PCR analysis of Plk1 levels in CD4+ T cells from SIV-infected and noninfected RM and SM was performed (Fig. 7). It can be seen that in RM high viral loads (viremia of >107 copies/ml) of SIV with concurrent AIDS symptomatology led to a four- to fivefold increase in the levels of Plk1 (P < 0.001). Interestingly, CD4+ T cells from SIV-positive but disease-resistant SM and CD4+ T cells from asymptomatically SIV-infected RM with low viremia (<106 copies/ml) did not lead to any significant changes in Plk1 levels. The moderate but significant increases in the levels of Plk1 message in symptomatically SIV-infected RM were also reflected at the protein level (Fig. 8A). It was of further interest to investigate whether symptomatic SIV infection exhibits a dysregulatory effect on Plk1 expression similar to the effect observed with Plk3. Cell cycle analysis of HSRM1 cells showed that, similarly to Plk3, the level of Plk1 expression is associated with the cell cycle progression (peaking in the M phase; Fig. 8B) and that CD3-CD28 stimulation of these cells leads to a gradual increase in Plk1 expression, peaking at the 24-h time point. Interestingly, similar stimulation of CD4+ T cells from SIV-infected RM led to a much slower increase in Plk1 levels that peaked at 48 h poststimulation. This slower response corresponded to the slow induction of cyclin B expression observed in these cells (Fig. 6). These data seem to suggest that, in the later symptomatic stage, pathogenic SIV infection has yet another effect on the polo-like kinase pathway manifested by an increase in baseline levels of Plk1 and an aberrant Plk1 response induced by cell stimulation.
FIG. 7.
SIV-induced upregulation of Plk1 correlates with viral load in RM. CD4+ T cells from SIV-seronegative SM (SM−), SIV-seropositive SM (SM+), SIV-negative RM (RM−), SIV-infected RM with a viral load of <106 vRNA copies/ml (RM+), and SIV-infected RM with a viral load of >107 vRNA copies/ml (RM++) were analyzed for the transcription of Plk1 by real-time PCR, and data were normalized to GAPDH and expressed as copy numbers relative to the calibrator sample. Error bars represent SD of values obtained from samples from different animals, and n denotes numbers of animals tested in each group. Statistically significant differences (asterisk; P < 0.001) were detected in RM++ samples compared to both RM+ samples and RM− samples.
FIG. 8.
Dysregulation of basal and stimulation-induced levels of Plk1 expression in CD4+ T cells from SIV-infected RM. (A) Lysates from CD4+ T cells purified from SIV-negative (SIV−) or SIV-positive (SIV+) RM were assayed for the expression of Plk1 by Western blotting; ID, integrated density. (B) HSRM1 cells synchronized at various stages of the cell cycle (Fig. 5) were assayed for the expression of Plk1 by Western blotting. Cells were either nonsynchronized (ctrl), synchronized at G1/S phase with aphidicolin (G1/S) or at G2/M phase with nocodazole (M), or assayed 2 h after the release of the nocodazole block (M+2). (C) HSRM1 cells and CD4+ T cells from SIV-infected RM were stimulated with anti-CD3-anti-CD28 antibody-coated beads for indicated time intervals and assayed for the expression of Plk1 by Western blotting. For primary CD4+ T cells representative data from a group of three animals are shown.
DISCUSSION
Previous work from several laboratories has documented the ability of HIV and SIV infection in vitro to induce G2/M cell cycle arrest. It was subsequently shown that it is both HIV- and SIV-derived Vpr, possibly acting through its interaction with the protein hVIP, that causes this arrest, which is characterized by the accumulation of hyperphosphorylated-inactive Cdc2 (1, 24, 32). It was also suggested that the underlying mechanism involves the inhibition of Cdc25C that subsequently fails to dephosphorylate-activate Cdc2. In contrast, studies with CD4+ T cells ex vivo—primary targets of lentivirus—collected from HIV-infected humans or SIV-infected nonhuman primates showed hyperactivation of Cdc2 with aberrant M-phase progression (7, 37, 39; B. Cervasi, M. Paiardini, B. Sumpter, G. Constantino, M. Magnani, G. Piedimonte, and G. Silvestri, 20th Annu. Symp. Nonhuman Primate Models AIDS, abstr. 75, 2002). It is important to note that results of recent in vitro studies suggest that the apoptosis following the G2/M arrest induced in vitro by Vpr is characterized by an increase of dephosphorylated-active Cdc2 caused by the depletion of Wee, a protein kinase that phosphorylates-inactivates Cdc2 (53).
Regulation of the G2/M and M-phase transition is a complex process involving activation of the cyclin B/Cdc2 complex (metaphase-promoting factor [MPF] complex) associated with G2/M progression, onset of mitosis, and subsequent degradation-inactivation of the MPF complex necessary for mitotic exit (reviewed in reference 28). The activation of Cdc2 is brought about by its dephosphorylation, which is regulated by phosphatase Cdc25C. The activation of Cdc25C is in turn regulated by, among others, the polo-like kinases Plk1 and Plk3 (36, 42). Plk1-mediated phosphorylation of Cdc25C on Ser198 leads to its nuclear translocation and activation while Plk3-mediated phosphorylation of Cdc25C on Ser216 leads to its inactivation-nuclear export. When inactivated, Cdc25C can no longer dephosphorylate-activate Cdc2, leading to the cell cycle progression from the M phase. Both Plk1 and Plk3 kinases provide other complex regulatory functions associated with mitosis (reviewed in reference 18). We showed previously and in the present study that Plk3 is markedly downregulated within CD4+ T cells from SIV-infected RM (Fig. 1). Similar downregulation of Plk3 was previously observed in various types of human malignant tumors, such as head, neck, and lung cancer cells (10, 11, 30). Downregulation of Plk3, therefore, clearly seems to be a marker of dysregulated cell proliferation under various pathogenic conditions, highlighted herein by its potential role in inducing the M-phase abnormalities observed in lentivirus infection. Interestingly, SIV infection of both CD4+ T cells and HOS-CD4-CCR5 cells in vitro showed a marked increase, rather than a decrease, in Plk3 levels. Surprisingly, while SIVmac251-infected CD4+ T cells showed a marked increase in Plk3 (Fig. 1), it was only the infection with either SIVsmΔB670 or highly pathogenic SIVsmPbj14 that led to the marked upregulation of Plk3 in HOS-CD4-CCR5 cells (Fig. 2). This would suggest that isolate-specific properties play an important role in each experimental setting-cell lineage. Taken together it is possible that during in vitro infection (with a high frequency of infected cells) a direct effect of virus replication or the expression of a particular viral protein, such as Vpr, without the influence of complex in vivo regulatory mechanisms of the immune system leads to the overexpression of Plk3. This in turn may lead to the inactivation of Cdc25C, its inability to activate Cdc2, and cell cycle arrest as observed with Vpr treatment in vitro (24, 40, 46). In addition it has been shown previously that Plk3 upregulation is characteristic of p53-induced cell cycle arrest as a response to DNA or chemical damage leading to the phosphorylation of p53 mediated by Plk3 (51, 52). Similarly, increased expression of Plk3 following in vitro infection could reflect an increase of apoptosis via the p53 pathway. On the other hand, Plk3 downregulation observed in lentivirus infection in vivo may be a consequence of the dysregulation of the CD4 T-cell compartment induced primarily by an indirect effect of infection on T-cell homeostasis (similar to the downregulation of Plk3 observed in some human tumors with complex cell cycle dysregulation).
The present study also shows that the levels of Plk3 in CD4+ T cells correlate with cell cycle progression and therefore are likely to be critical components of cell cycle regulation, rather than of other cell functions, such as adhesion or synaptic formation, described in other highly specialized cell lineages, e.g., macrophages and neural cells, respectively (23, 26). However, SIV infection, besides its ability to severely downregulate the basal expression of Plk3, also causes significant aberration in the kinetics of Plk3 regulation induced by TCR-mediated cell activation. A slower gradual increase of Plk3 peaking at 24 h in uninfected cells elicited by TCR stimulation is in contrast with a faster complex pattern of Plk3 induction, downregulation, and repeated induction observed in CD4 T cells from infected animals. This finding, together with the lack of its correlation with cyclin B expression, may provide an explanation for Cdc2 and Cdc25C activation abnormalities previously observed in CD4 T cells from HIV-infected individuals (7).
We have established a herpesvirus saimiri-transformed CD4+ T-cell line that has proven to be a very good resource especially in cell cycle analysis experiments. Characterization by protein kinase differential display and cell cycle gene expression array analysis showed that both baseline and activation-induced responses in these cells were similar to those seen in the parental CD4+ T-cell lineage. A time course of gradual increase of cyclin B expression following TCR and CD28 stimulation with a peak at 24 h reflecting the progression through the cell cycle was similar to the time course observed ex vivo in primary human CD4+ T cells from HIV-negative human volunteers (7). This cell line therefore closely mirrors events within primary cells and can serve as a valuable resource for future studies, alleviating the constraint associated with the limited supply of primary cells.
Finally, in addition to the downregulation of Plk3, pathogenic SIV infection seems to upregulate Plk1. Plk1 regulates the initiation of the M phase by phosphorylation of Cdc25C on Ser198 and cyclin B, leading to their activation and nuclear import (25, 48, 49). During the late M phase Plk1 activates the anaphase-promoting complex, which targets cyclin B to ubiquitination (29). During this step, Plk1 seems to be an essential regulatory element since the cells experimentally depleted of Plk1 have normal or hyperactivated MPF with a concurrent block in cyclin B degradation and separation of chromatids (31). Plk1 levels also oscillate during the cell cycle (14, 50), and downregulation of Plk1 leads to the loss of proliferative capacity in some cell lines (17) and G2 arrest (44). Our study shows that CD4+ T cells from RM that harbor high SIV viral loads (>107 copies/ml) and exhibit AIDS symptoms also show a moderate but consistent increase in baseline Plk1 levels, compared to those of both uninfected RM and SM and seropositive SM ex vivo. It is, however, difficult to distinguish whether this upregulation is induced by high virus replication or is a consequence of developing AIDS symptomatology or both. From this perspective there are also limitations in the comparison between the high-SIV-load-harboring RM and seropositive SM that, in general, harbor SIV viral loads within the range of 104 to 107 copies/ml (8; data not shown). However, in addition to the increased baseline levels of Plk1, the data also showed an aberrant activation pattern of Plk1 induction following TCR activation in the CD4+ T cells from symptomatically SIV-infected RM. A much slower activation-induced response in these cells correlates with the observed delay in the induction of cyclin B (Fig. 6 and 8). Therefore, this finding suggests yet another mechanism involved in the observed SIV-induced M-phase dysregulation in addition to Plk3.
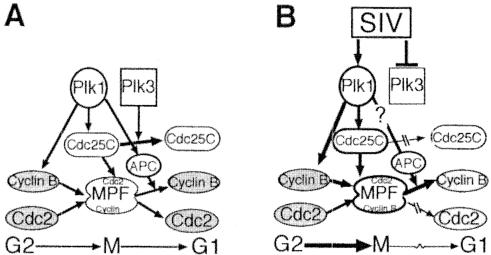
Based on the presented data, it seems feasible to propose the following model of the dysregulation leading to the M-phase irregularities in CD4+ T cells observed in chronic HIV and SIV infection (Fig. 9). G2/M-phase transition in CD4+ T cells is dependent on dephosphorylation-activation of Cdc2 and its association with cyclin B. In vivo chronic infection by SIV (and possibly HIV) leads to both marked downregulation of Plk3 and an aberrant Plk3 response after stimulation, which, in turn, leads to improper inactivation-degradation of Cdc25C and therefore increased and unscheduled activation of Cdc2. Furthermore, especially at later stages of SIV infection, disease-induced upregulation of Plk1 and aberrant Plk1 response lead to further dysregulation of activation of the MPF and the anaphase-promoting complex, leading to aberrations of both early and late M phase.
FIG. 9.
Effect of in vivo SIV infection on polo-like kinase pathway-mediated regulation of M phase. (A) Plk1- and Plk3-mediated regulation of M phase. (B) Proposed model of SIV infection-induced dysregulation of Plk pathway in vivo. APC, anaphase-promoting complex. Open shapes indicate active factors, and shaded shapes indicate inactive factors.
The mechanism of HIV-SIV-induced aberrations of M phase in vivo and in vitro is clearly a controversial issue that requires further studies. This study presents a novel mechanism of lentivirus-induced dysregulation in in vivo functioning upstream of those studied so far. In addition it also provides some insights into the pathogenesis of HIV- and SIV-induced defects within the CD4+ T-cell compartment. However, these mechanisms are complex and further studies are necessary to bring a detailed understanding of these complex regulatory effects.
Acknowledgments
This work was supported by NIH RO1 AI51994.
REFERENCES
- 1.Bartz, S. R., M. E. Rogel, and M. Emerman. 1996. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biesinger, B., I. Muller-Fleckenstein, B. Simmer, G. Lang, S. Wittmann, E. Platzer, R. C. Desrosiers, and B. Fleckenstein. 1992. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc. Natl. Acad. Sci. USA 89:3116-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostik, P., G. T. Brice, K. P. Greenberg, A. E. Mayne, F. Villinger, M. G. Lewis, and A. A. Ansari. 2000. Inverse correlation of telomerase activity/proliferation of CD4+ T lymphocytes and disease progression in simian immunodeficiency virus-infected nonhuman primates. J. Acquir. Immune Defic. Syndr. 24:89-99. [DOI] [PubMed] [Google Scholar]
- 4.Bostik, P., A. E. Mayne, F. Villinger, K. P. Greenberg, J. D. Powell, and A. A. Ansari. 2001. Relative resistance in the development of T cell anergy in CD4+ T cells from simian immunodeficiency virus disease-resistant sooty mangabeys. J. Immunol. 166:506-516. [DOI] [PubMed] [Google Scholar]
- 5.Bostik, P., P. Wu, G. L. Dodd, F. Villinger, A. E. Mayne, V. Bostik, B. D. Grimm, D. Robinson, H. J. Kung, and A. A. Ansari. 2001. Identification of protein kinases dysregulated in CD4+ T cells in pathogenic versus apathogenic simian immunodeficiency virus infection. J. Virol. 75:11298-11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brice, G. T., J. L. Riley, F. Villinger, A. Mayne, C. D. Hillyer, C. H. June, and A. A. Ansari. 1998. Development of an animal model for autotransfusion therapy: in vitro characterization and analysis of anti-CD3/CD28 expanded cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:210-220. [DOI] [PubMed] [Google Scholar]
- 7.Cannavo, G., M. Paiardini, D. Galati, B. Cervasi, M. Montroni, G. De Vico, D. Guetard, M. L. Bocchino, I. Picerno, M. Magnani, G. Silvestri, and G. Piedimonte. 2001. Abnormal intracellular kinetics of cell-cycle-dependent proteins in lymphocytes from patients infected with human immunodeficiency virus: a novel biologic link between immune activation, accelerated T-cell turnover, and high levels of apoptosis. Blood 97:1756-1764. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 74:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase, D., Y. Feng, B. Hanshew, J. A. Winkles, D. L. Longo, and D. K. Ferris. 1998. Expression and phosphorylation of fibroblast-growth-factor-inducible kinase (Fnk) during cell-cycle progression. Biochem. J. 333:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai, W., Y. Li, B. Ouyang, H. Pan, P. Reissmann, J. Li, J. Wiest, P. Stambrook, J. L. Gluckman, A. Noffsinger, and P. Bejarano. 2000. PRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancer. Genes Chromosomes Cancer. 27:332-336. [DOI] [PubMed] [Google Scholar]
- 11.Dai, W., T. Liu, Q. Wang, C. V. Rao, and B. S. Reddy. 2002. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int. J. Oncol. 20:121-126. [PubMed] [Google Scholar]
- 12.Donohue, P. J., G. F. Alberts, Y. Guo, and J. A. Winkles. 1995. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J. Biol. Chem. 270:10351-10357. [DOI] [PubMed] [Google Scholar]
- 13.Elder, R. T., M. Yu, M. Chen, X. Zhu, M. Yanagida, and Y. Zhao. 2001. HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology 287:359-370. [DOI] [PubMed] [Google Scholar]
- 14.Ferris, D. K., S. C. Maloid, and C. C. Li. 1998. Ubiquitination and proteasome mediated degradation of polo-like kinase. Biochem. Biophys. Res. Commun. 252:340-344. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher, T. M., III, B. Brichacek, N. Sharova, M. A. Newman, G. Stivahtis, P. M. Sharp, M. Emerman, B. H. Hahn, and M. Stevenson. 1996. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM). EMBO J. 15:6155-6165. [PMC free article] [PubMed] [Google Scholar]
- 16.Gale, M. J., Jr., J. A. Ledbetter, G. L. Schieven, M. Jonker, W. R. Morton, R. E. Benveniste, and E. A. Clark. 1990. CD4 and CD8 T cells from SIV-infected macaques have defective signaling responses after perturbation of either CD3 or CD2 receptors. Int. Immunol. 2:849-858. [DOI] [PubMed] [Google Scholar]
- 17.Georgescu, S. P., I. Komuro, Y. Hiroi, T. Mizuno, S. Kudoh, T. Yamazaki, and Y. Yazaki. 1997. Downregulation of polo-like kinase correlates with loss of proliferative ability of cardiac myocytes. J. Mol. Cell. Cardiol. 29:929-937. [DOI] [PubMed] [Google Scholar]
- 18.Glover, D. M., I. M. Hagan, and A. A. Tavares. 1998. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 12:3777-3787. [DOI] [PubMed] [Google Scholar]
- 19.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 20.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 21.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtrich, U., G. Wolf, A. Brauninger, T. Karn, B. Bohme, H. Rubsamen-Waigmann, and K. Strebhardt. 1994. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc. Natl. Acad. Sci. USA 91:1736-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtrich, U., G. Wolf, J. Yuan, J. Bereiter-Hahn, T. Karn, M. Weiler, G. Kauselmann, M. Rehli, R. Andreesen, M. Kaufmann, D. Kuhl, and K. Strebhardt. 2000. Adhesion induced expression of the serine/threonine kinase Fnk in human macrophages. Oncogene 19:4832-4839. [DOI] [PubMed] [Google Scholar]
- 24.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang, D., J. Chen, J. Wong, and G. Fang. 2002. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J. Cell Biol. 156:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauselmann, G., M. Weiler, P. Wulff, S. Jessberger, U. Konietzko, J. Scafidi, U. Staubli, J. Bereiter-Hahn, K. Strebhardt, and D. Kuhl. 1999. The polo-like protein kinases Fnk and Snk associate with a Ca2+- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 18:5528-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohn, E. A., N. D. Ruth, M. K. Brown, M. Livingstone, and A. Eastman. 2002. Abrogation of the S phase DNA damage checkpoint results in S phase progression or premature mitosis depending on the concentration of 7-hydroxystaurosporine and the kinetics of Cdc25C activation. J. Biol. Chem. 277:26553-26564. [DOI] [PubMed] [Google Scholar]
- 28.Kohn, K. W. 1999. Molecular interaction map of the mammalian cell cycle control and DNA repair systems. Mol. Biol. Cell 10:2703-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotani, S., S. Tugendreich, M. Fujii, P. M. Jorgensen, N. Watanabe, C. Hoog, P. Hieter, and K. Todokoro. 1998. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell 1:371-380. [DOI] [PubMed] [Google Scholar]
- 30.Li, B., B. Ouyang, H. Pan, P. T. Reissmann, D. J. Slamon, R. Arceci, L. Lu, and W. Dai. 1996. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J. Biol. Chem. 271:19402-19408. [DOI] [PubMed] [Google Scholar]
- 31.Liu, X., and R. L. Erikson. 2002. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc. Natl. Acad. Sci. USA 99:8672-8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahalingam, S., V. Ayyavoo, M. Patel, T. Kieber-Emmons, G. D. Kao, R. J. Muschel, and D. B. Weiner. 1998. HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc. Natl. Acad. Sci. USA 95:3419-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, R. H., and N. Sarver. 1997. HIV accessory proteins as therapeutic targets. Nat. Med. 3:389-394. [DOI] [PubMed] [Google Scholar]
- 34.Mori, K., Y. Yasutomi, S. Sawada, F. Villinger, K. Sugama, B. Rosenwith, J. L. Heeney, K. Uberla, S. Yamazaki, A. A. Ansari, and H. Rubsamen-Waigmann. 2000. Suppression of acute viremia by short-term postexposure prophylaxis of simian/human immunodeficiency virus SHIV-RT-infected monkeys with a novel reverse transcriptase inhibitor (GW420867) allows for development of potent antiviral immune responses resulting in efficient containment of infection. J. Virol. 74:5747-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nigg, E. A. 1998. Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol. 10:776-783. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang, B., W. Li, H. Pan, J. Meadows, I. Hoffmann, and W. Dai. 1999. The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene 18:6029-6036. [DOI] [PubMed] [Google Scholar]
- 37.Paiardini, M., D. Galati, B. Cervasi, G. Cannavo, L. Galluzzi, M. Montroni, D. Guetard, M. Magnani, G. Piedimonte, and G. Silvestri. 2001. Exogenous interleukin-2 administration corrects the cell cycle perturbation of lymphocytes from human immunodeficiency virus-infected individuals. J. Virol. 75:10843-10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng, C. Y., P. R. Graves, R. S. Thoma, Z. Wu, A. S. Shaw, and H. Piwnica-Worms. 1997. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277:1501-1505. [DOI] [PubMed] [Google Scholar]
- 39.Piedimonte, G., D. Corsi, M. Paiardini, G. Cannavo, R. Ientile, I. Picerno, M. Montroni, G. Silvestri, and M. Magnani. 1999. Unscheduled cyclin B expression and p34 cdc2 activation in T lymphocytes from HIV-infected patients. AIDS 13:1159-1164. [DOI] [PubMed] [Google Scholar]
- 40.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266-269. [DOI] [PubMed] [Google Scholar]
- 41.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roshak, A. K., E. A. Capper, C. Imburgia, J. Fornwald, G. Scott, and L. A. Marshall. 2000. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell. Signal. 12:405-411. [DOI] [PubMed] [Google Scholar]
- 43.Sabbaj, S., M. F. Para, R. J. Fass, P. W. Adams, C. G. Orosz, and C. C. Whitacre. 1992. Quantitation of antigen-specific immune responses in human immunodeficiency virus (HIV)-infected individuals by limiting dilution analysis. J. Clin. Immunol. 12:216-224. [DOI] [PubMed] [Google Scholar]
- 44.Smits, V. A., R. Klompmaker, L. Arnaud, G. Rijksen, E. A. Nigg, and R. H. Medema. 2000. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2:672-676. [DOI] [PubMed] [Google Scholar]
- 45.Stefanova, I., M. W. Saville, C. Peters, F. R. Cleghorn, D. Schwartz, D. J. Venzon, K. J. Weinhold, N. Jack, C. Bartholomew, W. A. Blattner, R. Yarchoan, J. B. Bolen, and I. D. Horak. 1996. HIV infection-induced posttranslational modification of T cell signaling molecules associated with disease progression. J. Clin. Investig. 98:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teeuwsen, V. J., K. H. Siebelink, F. de Wolf, J. Goudsmit, F. G. UytdeHaag, and A. D. Osterhaus. 1990. Impairment of in vitro immune responses occurs within 3 months after HIV-1 seroconversion. AIDS 4:77-81. [DOI] [PubMed] [Google Scholar]
- 48.Toyoshima-Morimoto, F., E. Taniguchi, and E. Nishida. 2002. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 3:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toyoshima-Morimoto, F., E. Taniguchi, N. Shinya, A. Iwamatsu, and E. Nishida. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215-220. [DOI] [PubMed] [Google Scholar]
- 50.Uchiumi, T., D. L. Longo, and D. K. Ferris. 1997. Cell cycle regulation of the human polo-like kinase (PLK) promoter. J. Biol. Chem. 272:9166-9174. [DOI] [PubMed] [Google Scholar]
- 51.Xie, S., Q. Wang, H. Wu, J. Cogswell, L. Lu, M. Jhanwar-Uniyal, and W. Dai. 2001. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J. Biol. Chem. 276:36194-36199. [DOI] [PubMed] [Google Scholar]
- 52.Xie, S., H. Wu, Q. Wang, J. P. Cogswell, I. Husain, C. Conn, P. Stambrook, M. Jhanwar-Uniyal, and W. Dai. 2001. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 276:43305-43312. [DOI] [PubMed] [Google Scholar]
- 53.Yuan, H., Y. M. Xie, and I. S. Chen. 2003. Depletion of Wee-1 kinase is necessary for both human immunodeficiency virus type 1 Vpr- and gamma irradiation-induced apoptosis. J. Virol. 77:2063-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]