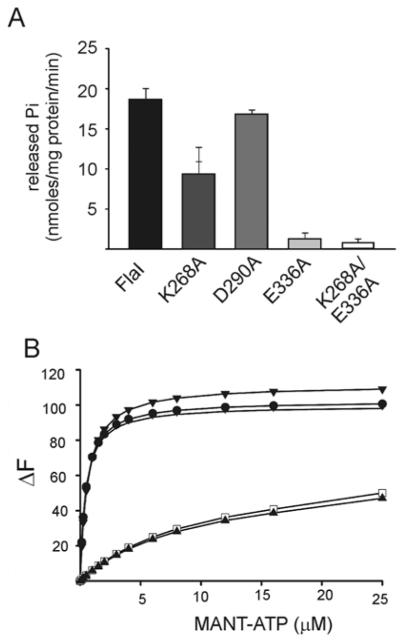
Figure 3. Characterization of ATP-binding and hydrolysis motifs in FlaI.
(A) Comparative ATP-hydrolysing activity of Walker A (FlaIK268A), aspartate box (FlaID290A), Walker B (FlaIE336A), and Walker A and B double mutant (FlaIK268A/E336A) motifs against the wt FlaI protein (black). (B) Analysis of the binding of MANT-ATP by wt FlaI and the mutants as monitored by FRET. The change in fluorescence (ΔF) upon MANT-ATP binding is shown as a function of the concentration of MANT-ATP. Fluorescence spectra were corrected for the fluorescence of unbound MANT-ATP. The Kd values were calculated for each of the mutants and wt FlaI. Closed circle, FlaI; closed triangle, FlaIK268A/E336A; open square, FlaIK268A; vertical stripe, FlaID290A; closed diamonds, FlaIE336A.