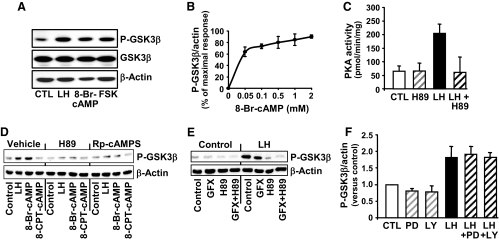
Figure 2.
The stimulatory response of LH on GSK3β phosphorylation (P-GSK3β) is mediated by cAMP/protein kinase (A). Primary cultures of steroidogenic luteal cells were treated as described below. Western blot analysis was performed to determine the levels of total GSK3β and GSK3β phosphorylated on ser9. β-Actin served as a loading control. A, Response to 15 min treatment with control media (CTL), LH (100 ng/ml), 8-Br-cAMP (1 mm), or forskolin (FSK; 10 μm). B, Concentration response to 15 min of treatment with 0.05–2 mm 8-Br-cAMP, means ± sem, n = 4. C, The stimulatory effect of LH on PKA activity is inhibited by the PKA inhibitor H89. Luteal cells were pretreated for 60 min with H89 (20 μm) before treatment for 15 min with LH (100 ng/ml). Protein kinase activity represents means ± sem from three separate experiments. D, PKA inhibitors prevent the stimulatory effects of LH and 8-Br-cAMP on GSK3β phosphorylation. Luteal cells were pretreated for 60 min with the PKA inhibitors H89 (20 μm) or Rp-cAMPS (0.5 μm) and then treated with LH (100 ng/ml), 8-Br-cAMP (1 mm) or 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′, 5′-cAMP (8-CPT-cAMP, 100 μm), a selective activator of EPAC but not PKA. E, Luteal cells were pretreated with the PKC inhibitor GF109203X (GFX; 10 μm), H89, or a combination of inhibitors for 60 min before treatment with LH (100/ml) for 15 min. F, Luteal cells were pretreated for 1 h with the MEK1/2 inhibitor PD98059 (PD; 50 μm) or the phosphatidylinositol 3-kinase inhibitor LY294002 (LY; 20 μm) before treatment with LH for 15 min. Results are means ± sem from three separate experiments.