Abstract
With each eye movement, the image received by the visual system changes drastically. To maintain stable spatiotopic (world-centered) representations, the relevant retinotopic (eye-centered) coordinates must be continually updated. Although updating or remapping of visual scene representations can occur very rapidly, J. D. Golomb, M. M. Chun, and J. A. Mazer (2008) demonstrated that representations of sustained attention update more slowly than the remapping literature would predict; attentional benefits at previously attended retinotopic locations linger after completion of the saccade, even when this location is no longer behaviorally relevant. The present study explores the robustness of this “retinotopic attentional trace.” We report significant retinotopic facilitation despite attempts to eliminate or reduce it by enhancing spatiotopic reference frames with permanent visual cues in the stimulus display and by introducing a different task where the attended location is the saccade target itself. Our results support and extend our earlier model of native retinotopically organized salience maps that must be dynamically updated to reflect the task-relevant spatiotopic location with each saccade. Consistent with the idea that attentional facilitation arises from persistent, recurrent neural activity, it takes measurable time for this facilitation to decay, leaving behind a retinotopic attentional trace after the saccade has been executed, regardless of conflicting task demands.
Keywords: coordinate systems, spatial attention, saccades, reference frame, remapping
Introduction
The world appears stable despite frequent eye movements that change the location of visual features on the retina. How does the visual system integrate constantly changing visual inputs while maintaining spatial attention across saccades? Information about exogenous visual stimuli and endogenously attended spatial locations must be combined at some level in the brain to generate the behavioral benefits associated with visual attention. One concept that has been discussed extensively in the literature is that these signals are merged in topographically organized visual maps, also known as salience maps (Koch & Ullman, 1985; Wolfe, 1994). Such representations, if retinotopic, require updating around the time of eye movements to ensure consistent, accurate allocation of attention. Representations of transient visual stimuli are thought to update before a saccade is even completed (Duhamel, Colby, & Goldberg, 1992; Heiser & Colby, 2006; Hunt & Cavanagh, 2009; Kusunoki & Goldberg, 2003; Melcher, 2007; Merriam, Genovese, & Colby, 2003, 2007; Nakamura & Colby, 2002; Parks & Corballis, 2008; Sommer & Wurtz, 2006), which is believed to smooth over the abrupt changes in retinal input generated by saccades. One aspect of updating that has been extensively studied is how quickly the visual representation of a stimulus presented before a saccade is “remapped” to its future location, i.e., where the representation would be located after the eye movement. However, the complementary process—how the representation at the previously relevant location decays—has been less explored. Kusunoki and Goldberg (2003) examined both the remapping and decaying processes for transient visual stimuli and found that responses to stimuli presented at the previous location began weakening before the eye movement, during the time the remapped location was coming online; stimuli presented after completion of the eye movement evoked no activity when presented in the previous location, indicating the neurons had completely updated their representations to the new location.
In contrast to such transient visual stimuli, endogenous sustained spatial attention appears to decay more slowly. In a set of recent psychophysical studies, Golomb, Chun, and Mazer (2008) explored how a locus of sustained spatial attention was updated after a saccade, whether facilitation persisted in retinotopic and/or spatiotopic coordinates, and whether these effects were influenced by task demands. Golomb et al. found that even when the task was designed to bias subjects toward maintaining locations in spatiotopic coordinates, after saccades attention initially persisted at task-irrelevant retinotopic coordinates. This residual retinotopic facilitation—detectable using both reaction time (RT) and accuracy measures—was maximal over the first 50–75 ms following the saccade and decayed back to control levels by 250 ms. Task-relevant spatiotopic facilitation was weaker under these experimental conditions but was sustained for several hundred milliseconds after the saccade, coexisting with the retinotopic facilitation at the early delays. Critically, when task demands were reversed to emphasize the retinotopic attentional representation, retinotopic facilitation was strong and persistent, and there was no evidence of spatiotopic facilitation at any delay. This asymmetry suggested that the two coordinate systems are not equivalent, and that the retinotopic coordinate system is the native system for visuospatial attention (Golomb et al., 2008). In other words, attentional loci are encoded and sustained in one or more retinotopically organized maps. Each eye movement requires updating of these retinotopic maps to reflect a new task-relevant retinal location, leaving the old retinotopic location facilitated for 100–200 ms after completion of the eye movement, even in the absence of any behavioral advantage.
This residual facilitation after the eye movement, termed here the “retinotopic attentional trace,” is likely to arise from the highly recurrent neural circuitry that instantiates regions of sustained spatial attention in retinotopically organized areas of visual cortex. Consistent with this model, the trace may reflect a fundamental property of sustained visuospatial attention, one that is largely impervious to changes in task demands. In the first experiment of the original report (Golomb et al., 2008), observers were biased toward a spatiotopic representation by asking them to perform an explicitly spatiotopic working memory task (for which the retinotopic location was task irrelevant) and by making spatiotopic probes twice as likely as retinotopic probes. Despite these explicit and implicit spatiotopic biases, facilitation was found at the task-irrelevant retinotopic location in addition to the task-relevant spatiotopic location immediately following a saccade. A subsequent experiment, described in the same report, demonstrated an absence of spatiotopic benefits when explicit and implicit task biases were shifted from spatiotopic to retinotopic representations, suggesting that the task manipulations were effective in directing observers to adopt either spatiotopic or retinotopic strategies.
However, one could argue that the spatiotopic cues and incentives in the spatiotopically biased task were simply not strong enough to suppress the retinotopic benefits. To further strengthen the spatiotopic bias, we considered a theory of visual stability across saccades which suggests that visual information present both before and after the saccade—e.g., the saccade target—can be used to compare views and anchor representations in a spatiotopic reference frame (Currie, McConkie, Carlson-Radvansky, & Irwin, 2000; McConkie & Currie, 1996). To examine the question of whether the retinotopic attention trace would persist when spatiotopic cues are further enhanced, Experiment 1 modified the original spatiotopically biased task by adding constantly present, fixed spatiotopic gridlines to the stimulus display to facilitate spatiotopic comparisons before and after saccades.
Additionally, we asked whether the retinotopic attentional trace is a fundamental property of sustained visuospatial attention or whether it is specific to the details of the particular attentional task described in the original experiment. The robust nature of the retinotopic attentional trace suggests that spatial attention primarily operates within the retinotopic coordinate system, but to date, our investigations have focused only on a specific type of spatial attention, namely, sustained attention directed toward an independent spatial location (i.e., one unrelated to the saccade target). However, visuospatial attention is usually allocated to saccade targets during oculomotor planning (Bisley & Goldberg, 2003; Gersch, Kowler, & Dosher, 2004; Hoffman & Subramaniam, 1995; Irwin & Gordon, 1998; Van der Stigchel & Theeuwes, 2005), possibly through a different neural circuit. In Experiment 2, we sought to determine whether the attentional facilitation associated with oculomotor planning also exhibits a similar retinotopic trace consistent with an underlying retinotopic coordinate system. In this experiment, we used a memory-guided saccade paradigm, where the attended location was the saccade endpoint. We investigated whether, after successful execution of a saccade to the cued location, attention would be restricted to the task-relevant spatiotopic target or whether an additional locus of attention would coexist at the task-irrelevant retinotopic location of the cue. If covert and overt attentional systems rely on the same mechanisms, as the premotor theory suggests (Rizzolatti, Riggio, Dascola, & Umilta, 1987), or if the retinotopic attentional trace is a general property of any type of sustained spatial attention, then we would expect attention under these conditions to also reside in retinotopically organized maps, leading to residual facilitation at the retinotopic location of the saccade target. On the other hand, the large body of literature on double-step saccades suggests that saccade targets can be successfully maintained in spatiotopic coordinates (Hallett & Lightstone, 1976; Mays & Sparks, 1980), and thus it is also possible that we would observe no retinotopic facilitation or perhaps even inhibition relative to equidistant control locations. Intriguingly, Abrams and Pratt (2000) found that the reference frames for inhibition of return differed according to whether covert or overt orienting was involved; they concluded that inhibition of eye movements occurred in retinotopic coordinates, while inhibition of spatial attention occurred in spatiotopic coordinates. If overt orienting is in fact more retinocentric than covert orienting, we may expect even stronger retinotopic effects here, although it is also possible that inhibition and sustained facilitation generally use reference frames differently. To explore this, Experiment 2 addresses whether the covert or ultimately overt nature of the task-relevant location influences the coordinate system in which it is maintained.
Methods
Subjects
Twenty subjects participated in each experiment (Experiment 1: 12 women; mean age, 22.1 years; range, 18–28 years; Experiment 2: 13 women; mean age, 21.4 years; range, 18–28 years). All subjects had normal or corrected-to-normal vision, and five subjects participated in both experiments. Every subject who participated was first prescreened in a no-saccade task to measure attentional facilitation in the absence of an eye movement and to ensure compatibility with the eye-tracker and confirm the subject's ability to maintain steady fixation (per Golomb et al., 2008). Because these experiments were designed specifically to investigate what happens to attentional facilitation after an eye movement, only individuals who exhibited attentional facilitation in the no-saccade task (i.e., during prescreening) were invited to participate as subjects in the actual experiments. Informed consent was obtained from all subjects, and the study protocol was approved by the Human Investigation Committee of the School of Medicine and the Human Subjects Committee of the Faculty of Arts and Sciences at Yale University. Subjects were compensated monetarily for their time.
Experimental setup
Stimuli were generated using the Psychtoolbox extension (Brainard, 1997) for Matlab (The Mathworks, Inc., Natick, MA) and were presented on a 19-in. flat-screen CRT monitor. Subjects were seated at a chin rest positioned 68 cm from the monitor so that the entire display subtended 25.7 × 19.2 degrees of visual angle. The experiments were performed in a dark room, with ambient illumination from the monitor and eye-tracking equipment. Eye position was monitored using an ISCAN eye-tracking system (ISCAN, Inc., Burlington, MA) recording pupil and corneal reflection (CR) position at 60 Hz; if gaze angle (pupil-CR) at any point during fixation deviated more than 2° from the center of the fixation dot, subjects received an error signal and the trial was aborted and repeated later in the run.
Eye tracking
At the beginning of each session, subjects performed an eye tracker calibration task, followed by a brief fixation practice task, which was used to verify calibration accuracy. These tasks were repeated if the calibration was inaccurate or became inaccurate at any point during the session. Eye-position data were acquired in real time during testing to generate gaze-contingent display changes and were also saved for subsequent offline analyses.
Task design
The tasks were modifications of the original “saccade” task described in Golomb et al. (2008). Subjects executed a guided saccade while sustaining attention at a cued spatiotopic spatial location. At various delays after the saccade, probe stimuli were presented at either the spatiotopic or retinotopic location of the cue or at an eccentricity-matched control location, and subjects made a speeded orientation discrimination response to the probe. Details of each task are described below. Probe delays, probe locations and saccade directions were always randomly intermixed and unpredictable; saccades could be executed in either horizontal or vertical directions (diagonal saccades were also included in Experiment 1). For each trial, fixation and cue positions were chosen at random from a list of possible configurations for the specified condition. If a trial was aborted due to a fixation break, it was replaced later in the run with a new probe location chosen at random, while delay and location (spatiotopic, retinotopic or control) were preserved.
Experiment 1
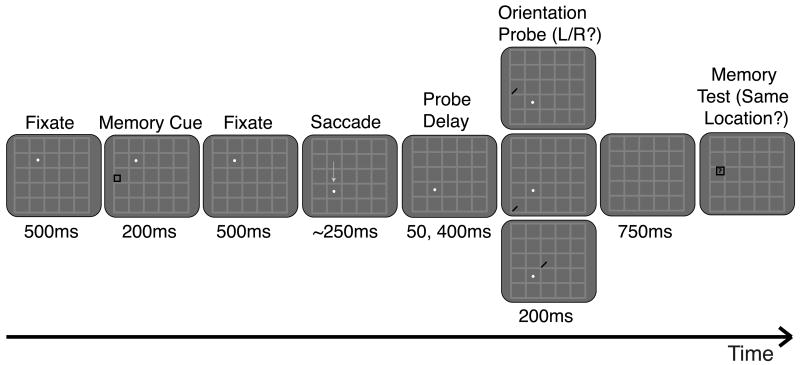
At the beginning of each trial, a white fixation dot (0.12° in diameter) appeared at one of four possible fixation locations, positioned at the corners of a 7° × 7° square (not visible) centered on the monitor. A faint gray 5 × 5 grid centered on these four locations was present on the screen at all times (Figure 1). Subjects initiated each trial by fixating on the fixation dot. After 500 ms, a memory cue (0.5° × 0.5° black square frame) appeared. Subjects were instructed to covertly attend to the location of the cue and to hold its spatiotopic coordinates (“exact screen location”) in memory throughout the trial. After 200 ms, the memory cue was removed, and subjects continued fixating for 500 ms. The fixation dot then disappeared and reappeared in a new fixation location, and subjects had 350 ms to execute a single accurate saccade and fixate this new location (while still attending/remembering the spatiotopic location of the memory cue).
Figure 1.
Task and conditions, Experiment 1. While subjects maintained fixation on the white fixation dot, a memory cue appeared briefly at another location. Subjects were instructed to hold this cued location in memory throughout the trial. The fixation dot then moved, and after completion of a saccade to the new fixation location, a probe stimulus (oriented bar) appeared after a variable delay in either the cue's spatiotopic (top), retinotopic (middle), or control (bottom) location. Subjects made a speeded button press response to indicate probe orientation. A memory test stimulus then appeared, and subjects indicated whether or not it occupied the same spatiotopic location as the memory cue. The light gray gridlines remained on the screen at all times to provide additional spatiotopic cues. The gray arrow depicting the saccade did not actually appear on the screen. Stimulus configuration illustrated here represents only one of several possible, counterbalanced cue-saccade configurations.
Once fixation was acquired at the new location, a probe stimulus (0.5° long black bar oriented 45° right or left of vertical) appeared after a delay of either 75 or 400 ms. The probe was presented for 200 ms and then extinguished. Subjects were instructed to report the orientation of the probe (left or right) as quickly as possible by pressing one of two keypad buttons. Half of the trials probed the memory task-relevant “spatiotopic” location, and the other half were evenly divided between “retinotopic” and “control” locations. As in prior studies, this 2:1 spatiotopic bias was included to further encourage subjects to maintain attention in the spatiotopic reference frame. Subjects completed at least 40 trials per condition (delay × location, where location was either spatiotopic, retinotopic, or control).
After subjects reported the probe orientation, there was a 750-ms interval before the fixation dot disappeared (freeing subjects to move their eyes) and a separate memory test stimulus appeared. The memory test stimulus (black square the same size as the cue) could appear in either the exact same spatiotopic location as the cue or in a neighboring location; distance between neighboring locations was adjusted dynamically (as detailed in Golomb et al., 2008) to keep memory test performance around 75% accuracy for each subject. Subjects were instructed to indicate by button press whether or not the memory test stimulus occupied the same exact spatiotopic location as the memory cue. An intertrial interval of 2000 ms (during which a uniform gray screen with only the gridlines present was displayed) preceded the start of the next trial.
Experiment 2
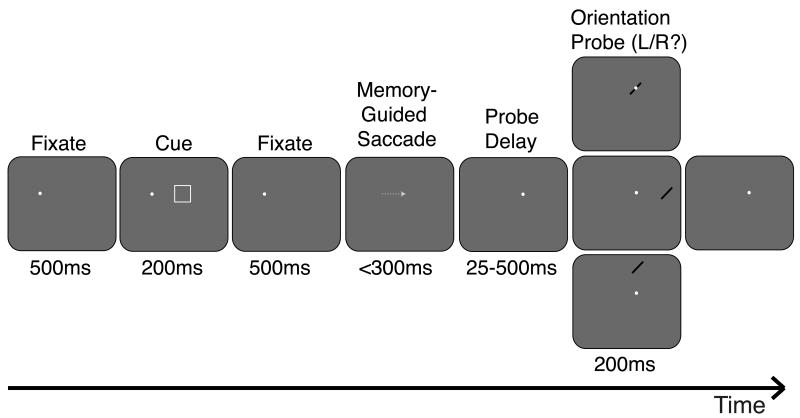
The stimulus configuration and task described for Experiment 1 were modified for the memory-guided saccade experiment (Figure 2). The four fixation locations were arranged as the corners of an invisible 5° × 5° square centered on the monitor. The smaller dimensions compared to Experiment 1 were necessary to fit all possible probe locations on the screen. Saccades were guided in only the horizontal or vertical directions for this task; no diagonal saccades were used. After a 500-ms fixation period, a memory cue (white square) appeared at one of the adjacent fixation locations for 200 ms. Subjects were instructed to covertly attend to the location of the cue and plan (but not yet execute) an eye movement to this location. After another 500 ms of fixation, the fixation dot disappeared, directing subjects to make a memory-guided saccade to the attended location. Subjects had only 300 ms to execute a single accurate saccade to the remembered location; this short time limit was adopted to encourage subjects to plan the eye movement in advance and thus keep attention focused at this location. Fixation breaks, inaccurate saccades, or premature saccades caused the trial to be aborted and repeated later in the run.
Figure 2.
Task and conditions, Experiment 2. While subjects maintained fixation on the white fixation dot, a cue appeared briefly indicating the target of the upcoming saccade. Subjects were instructed to plan an eye movement to this location but not to execute the eye movement until the fixation dot disappeared. Once the fixation dot disappeared, subjects had 300 ms to accurately complete the memory-guided saccade. Upon completion of the saccade, the fixation dot reappeared in the new location, and a probe stimulus (oriented bar) later appeared after a variable delay in either the cue's spatiotopic (top), retinotopic (middle), or control (bottom) location. Subjects made a speeded button press response to indicate probe orientation. The gray arrow indicating the saccade did not actually appear on the screen. Stimulus configuration illustrated here represents only one of several possible counterbalanced cue-saccade configurations.
Once the saccade was successfully completed, the fixation dot reappeared at the correct current fixation location. After a delay of 25, 75, or 500 ms, the probe stimulus (oriented bar) appeared in one of three locations: the spatiotopic location of the cue, which was also the current fixation location (50%), the retinotopic location of the cue (25%), or a control location of equal eccentricity to the retinotopic location (25%). Subjects completed at least 30 trials per condition (delay × location) over one to two sessions. Because it was not possible to eccentricity-match the spatiotopic location, which was always foveal after an accurate saccade, the critical comparison for this experiment was between retinotopic and eccentricity-matched control locations. There was no separate memory task for this experiment; successful (and rapid) completion of the memory-guided saccade ensured that subjects were attending to the spatiotopic location on each trial.
Analysis of attentional facilitation
Average reaction time (RT) on the speeded orientation discrimination report was calculated separately for each subject, location, and delay and submitted to random effects analyses. Trials in which the subject responded incorrectly on the probe orientation task or RT was greater than 3 standard deviations outside the subject's mean RT were excluded. Attentional facilitation was assessed in the same way as previously reported (Golomb et al., 2008): we took the differences in RT when the probe appeared in the spatiotopic or retinotopic locations compared to the control location baseline (positive differences reflect facilitation). The control baseline was calculated independently for each subject and delay, and the spatiotopic and retinotopic values were each compared to the same baseline since all three locations were equated for visual eccentricity. Repeated measures analyses of variance (ANOVAs) were conducted for each experiment with location and delay as within-subjects variables. If there were no significant main effects of or interactions with delay, we collapsed across delays and ran t-tests to assess whether spatiotopic or retinotopic locations were significantly facilitated compared to the control baseline (0-ms RT difference). If there were effects of delay, we conducted post hoc t-tests separately for each delay. All t-tests were paired and two-tailed.
Results
Experiment 1: Spatiotopic gridlines
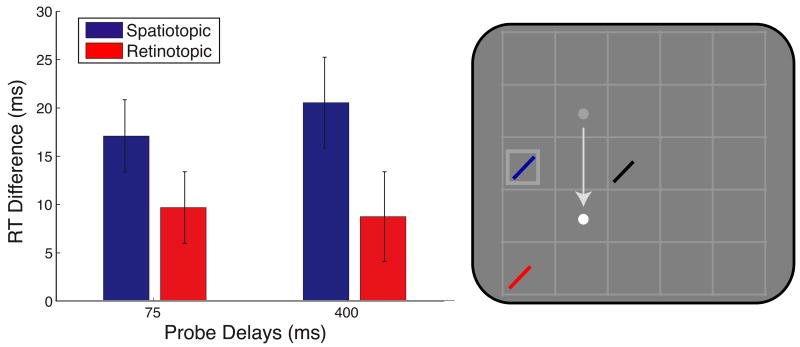
Figure 3 shows RT facilitation in Experiment 1 for spatiotopic and retinotopic probes appearing at 75 and 400 ms after the eye movement. There was no main effect of delay nor a Delay × Location interaction (both F's < 1); across delays, both spatiotopic and retinotopic locations were significantly facilitated over control (t19 = 2.85, p = 0.010, and t19 = 3.35, p = 0.003, respectively). There was a significant main effect of location (F1,19 = 4.38, p = 0.050), indicating that spatiotopic facilitation was stronger than retinotopic facilitation in this task. This greater facilitation at the task-relevant spatiotopic location compared to the retinotopic location across delays was not seen in the original experiments (Golomb et al., 2008), suggesting that the spatiotopic gridlines served to further reinforce the spatiotopic coordinate system. Despite this increase in task-relevant spatiotopic facilitation, there was still a significant retinotopic attentional trace.
Figure 3.
Attentional facilitation: Experiment 1. Attentional facilitation is plotted as the difference in RT for probes appearing in the spatiotopic and retinotopic locations compared to the control location baseline (zero). Positive values indicate attentional facilitation (RTs faster than at control locations). Data are plotted as a function of probe delay. Right panel illustrates sample probe locations for each experimental condition colored according to the plot legend on the left with black indicating the control location. White and gray dots indicate final and previous fixation locations, respectively; square indicates cued location, and arrow indicates saccade pattern. Error bars indicate standard error of the mean (SEM) after normalization to remove between-subject variability (Loftus & Masson, 1994); n = 20.
Experiment 2: Memory-guided saccade task
In Experiment 2, after the memory-guided saccade subjects were significantly faster to respond to probes appearing at the spatiotopic location compared to the control location (51.7-ms advantage; t19 = 6.94, p < 0.001). However, it is important to note that this highly facilitated location was not only both the saccade target and the task-relevant location but also the current fixation location when the probes appeared. Consequently, spatiotopic probes appeared at 0° retinal eccentricity, while the retinotopic and control probes appeared at 5° eccentricity. Visual eccentricity has a well-established impact on RT (Carrasco, Evert, Chang, & Katz, 1995), and in all of our previous experiments, the control location was carefully chosen to match for this important variable. Because there was no possible eccentricity-matched control location for a 0° probe, it is impossible to say how much of the spatiotopic benefit was due to attentional modulation and how much was due simply to enhanced visual sensitivity and processing at the fovea.
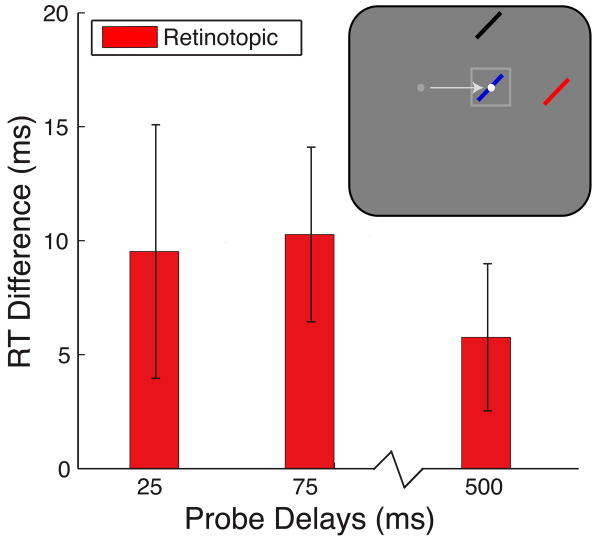
Thus, the critical comparison in Experiment 2 is whether the task-irrelevant retinotopic location of the saccade target was preferentially processed compared to the eccentricity-matched control location. Retinotopic facilitation at each delay is displayed in Figure 4. There was no significant main effect of delay (F < 1), although visual inspection of the data suggests that facilitation decreased at the later delay (500 ms) compared to the two earlier delays (25–75 ms). Overall, there was a significant benefit for the task-irrelevant retinotopic location over its eccentricity-matched control location (t19 = 2.88, p = 0.010). Thus, even when saccades were planned and successfully executed directly to the spatiotopic focus of attention, a retinotopic attentional trace still persisted at the previously attended retinotopic location.
Figure 4.
Attentional facilitation: Experiment 2. Attentional facilitation is plotted as the difference in RT for probes appearing at the retinotopic location of the cue compared to the control location baseline (zero). Positive values indicate attentional facilitation (RTs faster than at control locations). Data are plotted as a function of probe delay. Inset illustrates sample probe locations for each experimental condition with red indicating the retinotopic location, black indicating the control location and blue indicating the spatiotopic (current fixation) location. White and gray dots indicate final and previous fixation locations, respectively; square indicates cued location, and arrow indicates saccade pattern. Error bars are SEM after normalization to remove between-subject variability (Loftus & Masson, 1994); n = 20.
Discussion
These findings are consistent with our previous description of the retinotopic attentional trace (Golomb et al., 2008) and demonstrate its robustness across different task demands. In Experiment 1, despite successfully biasing the subjects even further toward maintaining attention in spatiotopic coordinates, we still found significant retinotopic facilitation. Not unexpectedly, the magnitude of the retinotopic facilitation under these conditions was reduced compared to the original studies. The observed lack of delay effects, however, was unexpected; retinotopic facilitation did not clearly decay as it had in previous studies. It is possible the gridlines provided some degree of retinotopic reference as well, although this possibility requires further investigation. Regardless, significant task-irrelevant retinotopic facilitation was clearly present in this task.
The retinotopic attentional trace seen in Experiment 2 was also considerably smaller than reported in our previous studies. This could be attributable to a number of factors. The most notable difference in Experiment 2 is that observers were only required to maintain the task-relevant location in memory until the eye movement, whereas in the previous studies the location remained task relevant until completion of the memory test, which occurred after the eye movement, presentation of the probe stimulus, and behavioral response to the probe. Changes to the interval over which subjects were required to sustain the attentional locus could affect the magnitude of attentional facilitation, i.e., in Experiment 2, subjects may have adopted a more transient attentional allocation strategy compared to the original task. In other words, if attentional facilitation before the eye movement was weaker, the retinotopic attentional trace should be weaker as well. Another difference in the current experiment is that the probes appeared at a smaller eccentricity (necessary to accommodate the modified spatial layout), and so the distance between probe positions was smaller. We have consistently found that the retinotopic attentional trace is greater with increasing eccentricity and distance between probe locations (Golomb et al., 2008, supplement and unpublished observations). Finally, it is possible that these effects reflect a veritable difference between tasks measuring covert and premotor spatial attention, and premotor representations may be inhibited or updated more effectively upon execution of the eye movement. Without an appropriate eccentricity-matched control, which is geometrically impossible, to properly evaluate spatiotopic facilitation, we cannot differentiate between these possibilities. Regardless, despite all of these factors that could be expected to reduce the magnitude of the retinotopic trace, we still found residual retinotopic facilitation after the saccade, demonstrating a high degree of resilience.
It should be noted that the memory-guided saccade task, by definition, necessitated that the retinotopic location fall in line with the saccade path. Because visual processing has been shown to be facilitated along the saccade path, particularly in the direction of the saccade (Gersch, Kowler, Schnitzer, & Dosher, 2009), we cannot rule out the possibility that saccade path enhancement effects might have contributed to the retinotopic advantage over control. However, we think it is unlikely that these effects completely account for our results, in light of the previous examples of the retinotopic attentional trace (Experiment 1 and Golomb et al., 2008) in which this effect was thoroughly controlled for. In Experiment 1, for example, although Figure 3 depicts a configuration in which the retinotopic location was in the direction of the saccade and control was located in the opposite direction, the diverse and unpredictable array of stimulus configurations ensured that on other trials it was equally likely that the opposite would be true or that both retinotopic and control locations would lie in the direction of the saccade. Thus, the retinotopic attentional trace is not simply a by-product of saccade-related facilitation.
These experiments attest to the robustness of the retinotopic attentional trace across task demands. We previously reported that loci of sustained spatial attention temporarily persist in retinotopic coordinates after a saccade (Golomb et al., 2008). This is in contrast to reports demonstrating that transient visual responses have both “remapped” to the new location (Duhamel et al., 1992; Heiser & Colby, 2006; Hunt & Cavanagh, 2009; Kusunoki & Goldberg, 2003; Melcher, 2007; Merriam et al., 2003, 2007; Nakamura & Colby, 2002; Parks & Corballis, 2008; Sommer & Wurtz, 2006) and extinguished at the previous location (Duhamel et al., 1992; Kusunoki & Goldberg, 2003) by the time the saccade is completed. We suggest that because sustained attentional facilitation has built up before the eye movement, it takes longer for this previous activation to decay, resulting in residual attentional “traces” that persist at the retinotopic location for a few hundred ms after the eye movement has completed. It should be noted that this attentional trace is not the same as a neural response to a stimulus presented before the saccade that persists for a brief period after the saccade (e.g., Heiser & Colby, 2006); the question is not whether the response itself persists, but whether a response is evoked (or enhanced) at all based on the attentional locus at the time of stimulus presentation.
The disparity between our results and the classic remapping literature suggests a difference between sustained and transient attention, a distinction previously made and proposed to engage different neural mechanisms (Corbetta & Shulman, 2002). We propose that spatial updating requires two complementary processes: remapping to the new location and deactivation or decay at the old location; the former may occur for any stimulus that is salient (Gottlieb, Kusunoki, & Goldberg, 1998), but the latter process is delayed when attention is sustained versus transient. This two-process updating system is similar to that thought to occur when spatial attention is covertly shifted to a new location in the absence of eye movements (Khayat, Spekreijse, & Roelfsema, 2006) and may also help explain the literature on inhibition of return, a phenomenon shown to have both spatiotopic and retinotopic components (Posner & Cohen, 1984; Sapir, Hayes, Henik, Danziger, & Rafal, 2004). Critically, when the parietal cortex, long thought to be involved in remapping (Duhamel et al., 1992; Heide, Blankenburg, Zimmermann, & Kömpf, 1995), is damaged or impaired, inhibition of return does not remap to the new spatiotopic location instead remains in retinotopic coordinates (Sapir et al., 2004; van Koningsbruggen, Gabay, Sapir, Henik, & Rafal, 2009).
As a further test of the generality of the retinotopic attentional trace, we employed a memory-guided saccade task in Experiment 2. The converging results suggest that sustained oculomotor attention shares similar mechanisms of updating with sustained covert attention. Although eye movements and covert shifts of attention evoke similar activity patterns in fMRI (Corbetta et al., 1998; Nobre, Gitelman, Dias, & Mesulam, 2000) and EEG (Eimer, Van Velzen, Gherri, & Press, 2007) and the premotor theory proposes that these two processes are one and the same (Rizzolatti et al., 1987), some studies have demonstrated dissociations (Hunt & Kingstone, 2003; Juan, Shorter-Jacobi, & Schall, 2004). Current thinking seems to be that attentional orienting and oculomotor planning share many overlapping mechanisms but are not always coactive (Awh, Armstrong, & Moore, 2006). The present study suggests that one of these overlapping mechanisms is retinotopically organized salience maps. This is particularly notable because of the ecological utility of double-step saccades and the ability to plan in advance a sequence of actions—in spatiotopic, real-world coordinates (Hallett & Lightstone, 1976). Of course, it should be noted that although there may be residual retinotopic facilitation after an eye movement, when the spatiotopic location is task relevant, attention does update to this location. In the current study as well as in several previous studies mentioned here, task-relevant spatiotopic facilitation coexists with the retinotopic attentional trace at the early delays. Even when early spatiotopic facilitation is not present and/or early retinotopic facilitation dominates, the task-relevant spatiotopic representation has been fully updated within a few hundred milliseconds after the saccade, a time scale that is consistent with recent reports exploring the updating of spatiotopic motor plans for double-step saccades (Bellebaum, Hoffmann, & Daum, 2005). Since humans typically make approximately three saccades per second (O'Regan, 1992), this may allow sufficient time for the updating process to complete, and the cost of encoding attention in retinotopic coordinates may not actually impair our ability to perform these tasks.
The current study demonstrates the robustness of the retinotopic attentional trace across multiple experimental paradigms and in the face of conflicting task demands. In Experiment 1, several factors combined to bias subjects against using a retinotopic attentional representation. Subjects were given explicit top–down instructions and feedback to maintain the spatiotopic representation. Implicitly, the probe was twice as likely to appear in the spatiotopic compared to retinotopic location. Finally, persistent visual cues remained on the screen at all times to help anchor subjects to spatiotopic representations. Local visual cues that are present both before and after the saccade should help the visual system achieve spatiotopic stability across saccades (McConkie & Currie, 1996). Although the saccade target may carry more weight in these comparisons (Currie et al., 2000), having spatiotopic gridlines constantly on the screen should also be expected to reinforce the updating process.
The presence of a retinotopic attentional trace in spite of all these spatiotopic cues provides further evidence that the underlying system for representing endogenously sustained visuospatial attention is retinotopic. Although we cannot generalize these findings across the full range of attentional tasks based on the experiments here, the robustness of the retinotopic attentional trace and its consistency with conventional neural models of attentional salience maps suggests that we may be tapping into a fundamental property of sustained visuospatial attention. We propose that the existence and magnitude of spatiotopic facilitation is dependent on its degree of task relevance, but the retinotopic attentional trace exists regardless of task demands or spatiotopic cues. Spatiotopic facilitation is only found when the spatiotopic representation is actively reinforced and is absent completely when retinotopic representations are task relevant (Golomb et al., 2008). The instability of spatiotopic attention may in part explain why several studies—measuring only spatiotopic facilitation—have concluded that it is not possible to maintain attention at one location while saccading elsewhere (Deubel & Schneider, 1996; Hoffman & Subramaniam, 1995; Kowler, Anderson, Dosher, & Blaser, 1995). In Experiment 1, the addition of constantly present gridlines reinforced the spatiotopic representations, and spatiotopic facilitation here exceeded the task-irrelevant retinotopic facilitation. Despite this increase in task-relevant spatiotopic facilitation, however, the retinotopic attentional trace was still present.
Across a wide variety of studies using different paradigms, evidence for both retinotopic and spatiotopic representations has been reported for trans-saccadic integration (Burr, Tozzi, & Morrone, 2007; Hayhoe, Lachter, & Feldman, 1991; Irwin, Brown, & Sun, 1988; Melcher, 2007; Melcher & Morrone, 2003; Prime, Niemeier, & Crawford, 2006; Watanabe & Suzuki, 1993), aftereffects (Afraz & Cavanagh, 2009; Ezzati, Golzar, & Afraz, 2008; Knapen, Rolfs, & Cavanagh, 2009; Melcher, 2005), and facilitation/inhibition of return (Abrams & Pratt, 2000; Posner & Cohen, 1984; Shimojo, Tanaka, & Watanabe, 1996). Some of these studies have reported effects in only one coordinate system or the other, while other reports have claimed both retinotopic and spatiotopic effects. While we suggest that the retinotopic coordinate system is the dominant system for sustained spatial attention, this should not be interpreted as saying that retinotopic facilitation should always exceed spatiotopic facilitation, even immediately after the saccade. The fact that the magnitude of spatiotopic and retinotopic facilitation could be modulated by task demands may help explain why the coordinate frame debate has remained ambiguous for these other studies; although the data presented here cannot speak as to whether the retinotopic coordinate system is actually dominant for these other tasks, it would be interesting to explore relative levels of retinotopic and spatiotopic effects when the task-relevant coordinate frame is varied.
The current study thus supports an attentional model of retinotopic facilitation and persistence: We have previously shown that attentional representations are encoded and maintained in retinotopic coordinates (Golomb et al., 2008); here we demonstrate the robustness of the resulting retinotopic attentional trace using additional tasks and imposing different behavioral demands. As a consequence, when a locus of attention is actively sustained, attentional facilitation lingers in retinotopic coordinates immediately after an eye movement, only updating to spatiotopic coordinates when behaviorally necessary.
Acknowledgments
This research was supported by research grants from NIH (R01-EY014193 and P30-EY000785 to M.M.C., F31-MH083374 to J.D.G), Whitehall Foundation to J.A.M., and NSF to J.D.G.
Footnotes
Commercial relationships: none.
Contributor Information
Julie D. Golomb, Interdepartmental Neuroscience Program, Yale University, New Haven, CT, USA
Vina Z. Pulido, Yale College, Yale University, New Haven, CT, USA
Alice R. Albrecht, Department of Psychology, Yale University, New Haven, CT, USA
Marvin M. Chun, Interdepartmental Neuroscience Program, Yale University, New Haven, CT, USA, Department of Psychology, Yale University, New Haven, CT, USA, & Department of Neurobiology, Yale University School of Medicine, New Haven, CT, USA
James A. Mazer, Interdepartmental Neuroscience Program, Yale University, New Haven, CT, USA, Department of Psychology, Yale University, New Haven, CT, USA, & Department of Neurobiology, Yale University School of Medicine, New Haven, CT, USA
References
- Abrams RA, Pratt J. Oculocentric coding of inhibited eye movements to recently attended locations. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:776–788. doi: 10.1037//0096-1523.26.2.776. [DOI] [PubMed] [Google Scholar]
- Afraz A, Cavanagh P. The gender-specific face aftereffect is based in retinotopic not spatiotopic coordinates across several natural image transformations. Journal of Vision. 2009;9(10):10, 1–17. doi: 10.1167/9.10.10. http://journalofvision.org/9/10/10/ [DOI] [PMC free article] [PubMed]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: Links, causes and implications for spatial attention. Trends in Cognitive Sciences. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Hoffmann KP, Daum I. Post-saccadic updating of visual space in the posterior parietal cortex in humans. Behavioural Brain Research. 2005;163:194–203. doi: 10.1016/j.bbr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Burr D, Tozzi A, Morrone MC. Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nature Neuroscience. 2007;10:423–425. doi: 10.1038/nn1874. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Evert DL, Chang I, Katz SM. The eccentricity effect: Target eccentricity affects performance on conjunction searches. Perception & Psychophysics. 1995;57:1241–1261. doi: 10.3758/bf03208380. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews, Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Currie CB, McConkie GW, Carlson-Radvansky LA, Irwin DE. The role of the saccade target object in the perception of a visually stable world. Perception & Psychophysics. 2000;62:673–683. doi: 10.3758/bf03206914. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Gherri E, Press C. ERP correlates of shared control mechanisms involved in saccade preparation and in covert attention. Brain Research. 2007;1135:154–166. doi: 10.1016/j.brainres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Golzar A, Afraz AS. Topography of the motion aftereffect with and without eye movements. Journal of Vision. 2008;8(14):23, 1–16. doi: 10.1167/8.14.23. http://journalofvision.org/8/14/23/ [DOI] [PubMed]
- Gersch TM, Kowler E, Dosher B. Dynamic allocation of visual attention during the execution of sequences of saccades. Vision Research. 2004;44:1469–1483. doi: 10.1016/j.visres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Gersch TM, Kowler E, Schnitzer BS, Dosher BA. Attention during sequences of saccades along marked and memorized paths. Vision Research. 2009;49:1256–1266. doi: 10.1016/j.visres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb JD, Chun MM, Mazer JA. The native coordinate system of spatial attention is retinotopic. Journal of Neuroscience. 2008;28:10654–10662. doi: 10.1523/JNEUROSCI.2525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Lightstone AD. Saccadic eye movements towards stimuli triggered by prior saccades. Vision Research. 1976;16:99–106. doi: 10.1016/0042-6989(76)90083-3. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Lachter J, Feldman J. Integration of form across saccadic eye movements. Perception. 1991;20:393–402. doi: 10.1068/p200393. [DOI] [PubMed] [Google Scholar]
- Heide W, Blankenburg M, Zimmermann E, Kömpf D. Cortical control of double-step saccades: Implications for spatial orientation. Annals of Neurology. 1995;38:739–748. doi: 10.1002/ana.410380508. [DOI] [PubMed] [Google Scholar]
- Heiser LM, Colby CL. Spatial updating in area LIP is independent of saccade direction. Journal of Neurophysiology. 2006;95:2751–2767. doi: 10.1152/jn.00054.2005. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Perception & Psychophysics. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Cavanagh P. Looking ahead: The perceived direction of gaze shifts before the eyes move. Journal of Vision. 2009;9(9):1, 1–7. doi: 10.1167/9.9.1. http://journalofvision.org/9/9/1/ [DOI] [PMC free article] [PubMed]
- Hunt AR, Kingstone A. Covert and overt voluntary attention: Linked or independent? Brain Research. Cognitive Brain Research. 2003;18:102–105. doi: 10.1016/j.cogbrainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Irwin DE, Brown JS, Sun JS. Visual masking and visual integration across saccadic eye movements. Journal of Experimental Psychology: General. 1988;117:276–287. doi: 10.1037//0096-3445.117.3.276. [DOI] [PubMed] [Google Scholar]
- Irwin DE, Gordon RD. Eye movements, attention and trans-saccadic memory. Visual Cognition. 1998;5:127–155. [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proceedings of National Academy of Sciences of the United States of America. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat PS, Spekreijse H, Roelfsema PR. Attention lights up new object representations before the old ones fade away. Journal of Neuroscience. 2006;26:138–142. doi: 10.1523/JNEUROSCI.2784-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen T, Rolfs M, Cavanagh P. The reference frame of the motion aftereffect is retinotopic. Journal of Vision. 2009;9(5):16, 1–7. doi: 10.1167/9.5.16. http://journalofvision.org/9/5/16/ [DOI] [PubMed]
- Koch C, Ullman S. Shifts in selective visual attention: Towards the underlying neural circuitry. Human Neurobiology. 1985;4:219–227. [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Research. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Goldberg ME. The time course of perisaccadic receptive field shifts in the lateral intraparietal area of the monkey. Journal of Neurophysiology. 2003;89:1519–1527. doi: 10.1152/jn.00519.2002. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Mays LE, Sparks DL. Saccades are spatially, not retinocentrically, coded. Science. 1980;208:1163–1165. doi: 10.1126/science.6769161. [DOI] [PubMed] [Google Scholar]
- McConkie GW, Currie CB. Visual stability across saccades while viewing complex pictures. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:563–581. doi: 10.1037//0096-1523.22.3.563. [DOI] [PubMed] [Google Scholar]
- Melcher D. Spatiotopic transfer of visual-form adaptation across saccadic eye movements. Current Biology. 2005;15:1745–1748. doi: 10.1016/j.cub.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Melcher D. Predictive remapping of visual features precedes saccadic eye movements. Nature Neuroscience. 2007;10:903–907. doi: 10.1038/nn1917. [DOI] [PubMed] [Google Scholar]
- Melcher D, Morrone MC. Spatiotopic temporal integration of visual motion across saccadic eye movements. Nature Neuroscience. 2003;6:877–881. doi: 10.1038/nn1098. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Spatial updating in human parietal cortex. Neuron. 2003;39:361–373. doi: 10.1016/s0896-6273(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Remapping in human visual cortex. Journal of Neurophysiology. 2007;97:1738–1755. doi: 10.1152/jn.00189.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4026–4031. doi: 10.1073/pnas.052379899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: Overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- O'Regan JK. Solving the “real” mysteries of visual perception: The world as an outside memory. Canadian Journal of Psychology. 1992;46:461–488. doi: 10.1037/h0084327. [DOI] [PubMed] [Google Scholar]
- Parks NA, Corballis PM. Electrophysiological correlates of presaccadic remapping in humans. Psychophysiology. 2008;45:776–783. doi: 10.1111/j.1469-8986.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. Attention & Performance X. 1984:531–556. [Google Scholar]
- Prime SL, Niemeier M, Crawford JD. Transsaccadic integration of visual features in a line intersection task. Experimental Brain Research. 2006;169:532–548. doi: 10.1007/s00221-005-0164-1. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Sapir A, Hayes A, Henik A, Danziger S, Rafal R. Parietal lobe lesions disrupt saccadic remapping of inhibitory location tagging. Journal of Cognitive Neuroscience. 2004;16:503–509. doi: 10.1162/089892904323057245. [DOI] [PubMed] [Google Scholar]
- Shimojo S, Tanaka Y, Watanabe K. Stimulus-Driven facilitation and inhibition of visual information processing in environmental and retinotopic representations of space. Brain Research. Cognitive Brain Research. 1996;5:11–21. doi: 10.1016/s0926-6410(96)00037-7. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Theeuwes J. The influence of attending to multiple locations on eye movements. Vision Research. 2005;45:1921–1927. doi: 10.1016/j.visres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Van Koningsbruggen MG, Gabay S, Sapir A, Henik A, Rafal RD. Hemispheric asymmetry in the remapping and maintenance of visual saliency maps: A TMS study. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21356. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Suzuki N. Visual integration during saccadic and pursuit eye movements: The importance of spatial framework. Perceptual and Motor Skills. 1993;77:1219–1234. doi: 10.2466/pms.1993.77.3f.1219. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0 A revised model of visual search. Psychonomic Bulletin & Review. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]