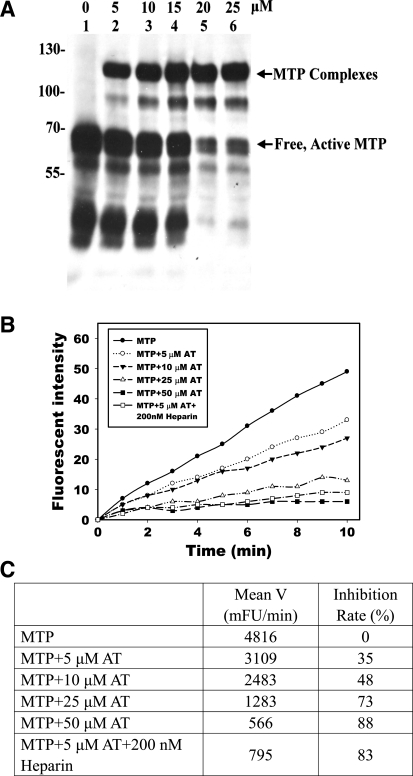
Fig. 6.
Complex formation and inhibition of active MTP by AT in vitro. Purified active MTP was incubated with increasing amounts of human AT, as indicated, and analyzed for the formation of MTP complexes with AT by immunoblotting using MTP mAb M24 (A) and for inhibition of MTP amidolytic activity using synthetic fluorescent substrate Boc-Gln-Ala-Arg-AMC (B and C). The effect of heparin (200 nM) on MTP inhibition was also determined. The proteolytic activities for the samples were determined by the rates of cleavage of the MTP substrate as indicated by the plots of the release of AMC (Fluorescent Intensity) against time (B). The inhibition was calculated by comparing the mean velocities (Mean V) of inhibitor-treated samples with MTP alone (C).