Abstract
Using a combination of wild-type (WT) and caveolin-2 (Cav-2) knockout along with retroviral reexpression approaches, we provide the evidence for the negative role of Cav-2 in regulating anti-proliferative function and signaling of transforming growth factor β (TGF-β) in endothelial cells (ECs). Although, TGF-β had a modest inhibitory effect on WT ECs, it profoundly inhibited proliferation of Cav-2 knockout ECs. To confirm the specificity of the observed difference in response to TGF-β, we have stably reexpressed Cav-2 in Cav-2 knockout ECs using a retroviral approach. Similar to WT ECs, the anti-proliferative effect of TGF-β was dramatically reduced in the Cav-2 reexpressing ECs. The reduced anti-proliferative effect of TGF-β in Cav-2-positive cells was evidenced by three independent proliferation assays: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), cell count, and bromodeoxyuridine incorporation and correlated with a loss of TGF-β-mediated upregulation of cell cycle inhibitor p27 and subsequent reduction of the levels of hyperphosphorylated (inactive) form of the retinoblastoma protein in Cav-2 reexpressing ECs. Mechanistically, Cav-2 inhibits anti-proliferative action of TGF-β by suppressing Alk5-Smad2/3 pathway manifested by reduced magnitude and length of TGF-β-induced Smad2/3 phosphorylation as well as activation of activin receptor-like kinase-5 (Alk5)-Smad2/3 target genes plasminogen activator inhibitor-1 and collagen type I in Cav-2-positive ECs. Expression of Cav-2 does not appear to significantly change targeting of TGF-β receptors I and Smad2/3 to caveolar and lipid raft microdomains as determined by sucrose fractionation gradient. Overall, the negative regulation of TGF-β signaling and function by Cav-2 is independent of Cav-1 expression levels and is not because of changing targeting of Cav-1 protein to plasma membrane lipid raft/caveolar domains.
Keywords: caveolin-1, activin receptor-like kinase-5, Smad2/3, plasminogen activator inhibitor-1, collagen type-1
caveolins are key components of detergent-resistant cholesterol lipid-rich membranes including lipid rafts and caveolae. Three members were identified within the caveolin protein family: caveolin-1 (Cav-1), Cav-2, and Cav-3 (53). Cav-1 and -2 are ubiquitously coexpressed, whereas Cav-3 is muscle specific (53). Cav-2 interacts with Cav-1 and forms hetero-oligomeric complexes within the caveolae (5, 46). The interaction with Cav-1 is required for transport of Cav-2 to the cell surface (27, 36). In the absence of Cav-1, Cav-2 is degraded, and its expression is markedly reduced (7, 38). Caveolins play numerous important roles. In addition to being key structural proteins that organize caveolae, caveolin proteins are important in regulating endocytosis and various aspects of cellular signaling (20, 34, 53). Although relative to Cav-1, the functional role of Cav-2 is less well defined, recent studies have started to present a growing body of evidence suggesting tissue/cell-specific role for Cav-2. For example, initial observations involving Cav-2 knockout (KO) mice revealed hyperplasia in the lung (39), suggesting a role for Cav-2 in regulating lung cell proliferation and/or differentiation. More recent studies discovered skeletal muscle abnormalities in Cav-2 KO mice, including mitochondrial aggregation and increased numbers of M-cadherin-positive satellite cells (42). Studies on the functional role of Cav-2 using overexpression and/or smalling interfering RNA (siRNA)/short hairpin RNA (shRNA) approaches imply that Cav-2 is involved in caveolae formation in epithelial cells (ECs) (8, 23, 46). Furthermore, Cav-2 appears to facilitate infection of mammalian cells with Pseudomonas aeruginosa (56, 57) and Rickettsia conorii (4). Cav-2 has also been shown to regulate endocytosis and trafficking of the M1 muscarinic receptor in Madin-Darby canine kidney cells (45) and apical lipid trafficking in the intestine of Caenorhabditis elegans (35). There is also evidence for a role of Cav-2 in regulating proliferation and STAT3 signaling in rat fibroblast cell line Hirc-B (19, 21, 22). More recently, we have shown that Cav-2 also regulates proliferation in lung ECs (55).
Transforming growth factor-β (TGF-β) is a multifunctional dimeric polypeptide growth factor capable of regulating proliferation, differentiation, migration, extracellular matrix production, and survival of various cell types. Cell responses to TGF-β are mediated through specific transmembrane type I and type II Ser/Thr kinase receptors (26, 48). The signaling pathway is initiated by TGF-β binding to the TGF-β type II receptor (TβR-II). Upon ligand binding, TβR-II recruits and phosphorylates TβR-I, also known as activin receptor-like kinase (Alk), which transduces the signal to the nucleus through members of the Smad family (16, 28). Most cell types express a form of TβR-I known as Alk5. ECs also coexpress an additional TβR-I known as Alk1. Interestingly, activated Alk5 induces the phosphorylation of Smad2 and Smad3, whereas activated Alk1 has been shown to induce the phosphorylation of Smad1 and Smad5 (10, 32, 33). The outcome resulting from the activation of these two major Smad-mediated signaling pathways is different. The activation of Alk5-Smad2/3 pathway leads to inhibition of cell proliferation and is associated with a mature endothelium with increased expression of genes such as plasminogen activator inhibitor-1 (PAI-1), collagen type I (Col 1), or fibronectin. Conversely, Alk1-Smad1/5 activates cell proliferation and migration and is more related to the angiogenic state with the expression of inhibitor of DNA binding 1 (Id-1) and endoglin, among others (3, 9, 11, 54).
There are several reports suggesting that some components of TGF-β signaling could localize to caveolae or interact with Cav-1 (6). However, no data linking Cav-2 to TGF-β signaling and function are available. Thus the goal of the present study was to determine whether Cav-2 expression regulates TGF-β-mediated signaling and function in ECs. We have focused on EC proliferation because it is very important for angiogenesis and can be regulated by TGF-β. Our data suggest that Cav-2 negatively regulates TGF-β-Alk5-Smad 2/3 pathway manifested by the reduction of an anti-proliferative effect of TGF-β in ECs. Since both Cav-2 and TGF-β functions are cell/tissue and context specific, our data should help to further advance understanding of the mechanistic basics of this specificity.
MATERIALS AND METHODS
Antibodies and reagents.
Antibodies against total Cav-2, Cav-1, and Hsp-90 were from BD Transduction. Phospho-serine 23-Cav-2 antibody was previously generated and characterized for immunofluorescence staining in our laboratory (47). Antibodies to cdk inhibitor p27Kip1 and total Smad1/5/8 were from Santa Cruz Biotech. Phospho- and total Smad2 and 3, phospho-Smad1/5/8, phospho-(serine 780) Rb, phospho-(threonine 202/tyrosine 204) ERK1/2, total ERK1/2, phospho-Akt, and total Akt were from Cell Signaling Biotech. TGF-β1 was from Peprotech, and SB-505124 (SB-5), an inhibitor of Alk4/5/7 (13), was from Sigma.
Cells.
Mouse lung endothelial cells (MLECs) were isolated from 2- to 3-wk-old wild-type (WT) and Cav-2 KO mice as previously described (55). Use of animals for this study was approved by the University of Missouri and the Thomas Jefferson University Animal Care and Use Committees. Briefly, mice were euthanized with an overdose of ketamine-xylazine, and the lungs were excised, minced, and digested with 0.1% collagenase in RPMI medium. The digest was homogenized by passing multiple times through a 14-gauge needle, filtered through 70-μm cell strainers, and the cell suspension plated on 0.1% gelatin-coated dishes. After 2 to 3 days, cells were immortalized by two rounds of infection with retrovirus encoding the polyoma middle T antigen. Cells were allowed to recover for 24 h, and then MLECs were isolated by immunoselection with PECAM-1-conjugated magnetic beads. When cells reached confluence, a second round of immunoisolation was performed. Cells were propagated in 40% DMEM (low glucose)/40% F12 HAM, 20% FBS, heparin (100 μg/ml), l-glutamine, and penicillin-streptomycin.
Primary human umbilical vein endothelial cells (HUVEC) were obtained from Lonza and maintained in culture in EGM2-MV medium (Lonza) and used for experiments at passage 3–4.
Retroviral reexpression of Cav-2 in Cav-2 KO MLECs.
DNA encoding human Cav-2, codon optimized for expression in mouse, was synthesized and subcloned into pUC57 vector by GenScript. The Cav-2 encoding DNA sequence was then transferred to retroviral expression vector pBABE-puro (deposited by Dr. Robert Weinberg; Addgene plasmid 1764) using standard subcloning procedure with EcoRI and SalI restriction enzymes.
Mouse-specific retroviral particles were generated by transfecting EcoPack2–293 packaging cell line (Clontech) with pBABE-puro without or with Cav-2 sequence. Viral supernatants were collected 2 days after transfection and used to infect passage 1 of proliferating Cav-2 KO MLECs in the presence of polybrene (8 μg/ml; Sigma) to facilitate viral infection. Two days after the third infection, cells were expanded in the presence of puromycin (5 μg/ml) until nearly 100% Cav-2-positive cells were obtained as determined by immunofluorescence labeling with anti-Cav-2 antibody (Fig. 2D).
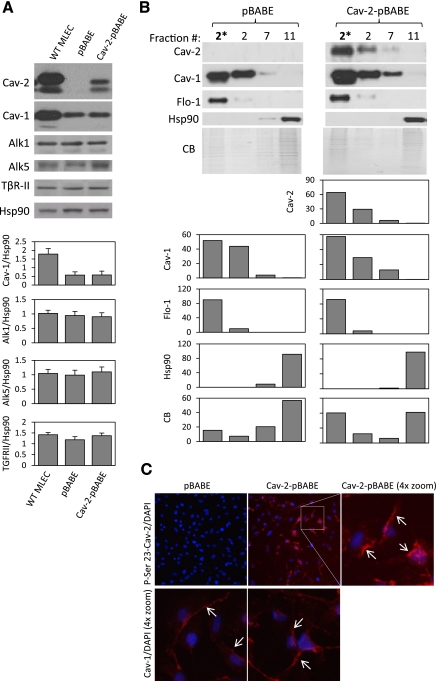
Fig. 2.
Reexpression of human Cav-2 in Cav-2 KO MLECs. Cav-2 KO MLECs were serially infected (total of 3 rounds) with retrovirus expressing an empty vector pBABE-puro (pBABE) or pBABE plus human Cav-2 (Cav-2-pBABE), followed by selection in the presence of puromycin as described in materials and methods. A: WT MLECs and Cav-2 KO MLECs expressing pBABE or Cav-2-pBABE using retroviral approach were lysed and immunoblotted with indicated antibodies. Graphs, densitometric ratios of Cav-1 and indicated TGF-β receptors (TβRs) to heat shock protein 90 (Hsp90) assessed using ImageJ as described in materials and methods. Note that low expression level of retrovirally delivered human Cav-2 compared with mouse Cav-2 expressed in WT MLECs (top immunoblot; lane 3 vs. lane 1 and top graph) is insufficient to increase the expression levels of endogenous Cav-1, which is considerably lower in pBABE and Cav-2-pBABE compared with WT MLECs (2nd immunoblot and top graph). B: pBABE and Cav-2-pBABE cells were lysed with TX-100, and lysates were subjected to sucrose floatation gradient as described in materials and methods, followed by immunoblotting of selected fractions with indicated antibodies to Cav-2, Cav-1, and Flo-1 (caveolar/lipid raft marker) as well as Hsp90 (cytosolic and heavy membrane marker). CB, Coomassie Blue staining of the above immunoblotted fractions for comparison of protein loading (the picture was compressed by threefold in the vertical dimension). Note that retrovirally expressed human Cav-2 cofractionates with Cav-1 and Flo-1 in caveolar/lipid raft fraction 2. Also, note that endogenous Cav-1 cofractionates with Flo-1 but not with Hsp90 in both pBABE and Cav-2-pBABE cells. 2*, concentrated fraction 2. Graphs, relative (%) distribution of densitometric signal values between selected fractions. C, top: immunofluorescence costaining with rabbit polyclonal antibody against phospho-serine 23-Cav-2 (P-Ser 23-Cav-2) and nuclear staining with DAPI (blue). Note, P-Ser 23-Cav-2-positive staining in Cav-2-pBABE (top middle and right) and lack of the respective staining in pBABE cells (top left). Also, note that retrovirally expressed P-Ser 23-Cav-2 is primarily localized in plasma membrane (arrows) (top right panel with 4× zoom). All microscopic images were captured using objective with ×20 magnification and a selected field depicted by a square in top middle panel was zoomed by 4× and shown in top right panel to visualize more detailed subcellular localization. C, bottom: immunofluorescence costaining with rabbit polyclonal antibody against Cav-1 and nuclear staining with DAPI (blue). Note that Cav-1 is primarily localized to plasma membrane (depicted by arrows) in both pBABE and Cav-2-pBABE cells (Fig. 2C; bottom left vs. right). Both images were captured using objective with ×20 magnification and further zoomed by 4× relative to respective top left and middle panels to show more detailed subcellular localization of Cav-1 similar to P-Ser 23-Cav-2 (shown in 2C, top right).
siRNA knockdown of Cav-2 in HUVEC.
Stealth siRNA targeting nuclotides 399–423 of human Cav-2 (GAAGAGTGTGACAGATGTTATCATT) and nonsilencing control siRNA (GAGAAGGTTGCATGAGTATACTATT) were obtained from Invitrogen. Briefly, HUVEC were transfected with 10 nM of each siRNA using siPORT amine (Ambion) according to manufacturer's protocol. Three days later, cells were treated without or with TGF-β for 1 h and lysed for immunoblotting.
MTT colorimetric proliferation assay.
MLECs were seeded onto 24-well plates at 4 × 103/well in complete medium. Next day, medium was replaced with fresh medium without or with TGF-β or SB-5. After 6 h (day 0) or 2–8 days of incubation in the absence or presence of TGF-β (0.01–10 ng/ml) or SB-5 (1 μM), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) stock solution was added to each well at a final concentration of 0.5 mg/ml and incubated at 37°C for 2 h, followed by medium removal and overnight drying and lysis with 0.5 ml of DMSO (Fisher) to solubilize the final product of MTT metabolism, formazan precipitate. Samples of 150-μl each were transferred to 96-well plates, and the optical density (OD) at 570 nm minus 750 nm was determined using microplate reader (Bio-Rad). The data are expressed as means ± SD (n = 3) values from one representative out of total four experiments. For OD higher than 1, samples were further diluted with DMSO, remeasured, and the final OD values calculated based on dilution factor.
Cell counts-based proliferation assay.
MLECs were plated onto 100-mm dishes at 105 cells per dish and incubated in complete medium in the absence or presence of TGF-β. On days 2, 4, 6, and 8, cells were trypsinized and viable cells counted with an automatic cell counter Countess (Invitrogen). Cell number was expressed as mean from one representative out of a total three experiments.
Bromodeoxyuridine labeling.
To determine the S-phase fraction (DNA synthesizing cells), proliferating MLECs treated for 3 days in the absence or presence of TGF-β (1 ng/ml) were incubated with 5-bromo-2-deoxyuridine (BrdU; 50 μg M) for 2 h, followed by fixing and denaturing DNA with 2 N HCl, and costaining with fluorescein isothiocyanate conjugated anti-BrdU antibody and 4′-6-diamidino-2-phenylindole (DAPI). BrdU-positive and total cells (stained with DAPI) were examined in nine randomly chosen independent fields using fluorescent microscope, and the data were expressed as mean %BrdU-positive cells ± SD (n = 9) values from one representative out of total three experiments.
Immunofluorescence microscopy.
Cells were fixed with 3% paraformaldehyde in Dulbecco's phosphate-buffered saline, pH 7.4 (DPBS), for 30 min, and washed three times with DPBS. Cells were then incubated sequentially with 0.1% Triton X-100 (vol/vol) in DPBS for 10 min, DPBS plus 5% goat serum for 30 min, and thereafter with appropriate antibodies in 0.2% BSA for 2 h, washed three times, and incubated with fluorescein isothiocyanate- or Texas Red-labeled secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA) diluted 1:500, followed by staining with DAPI. Slides were mounted with Slowfade (Molecular Probes, Eugene, OR), and cells were observed and images captured with ×20 objective using an Olympus IX70 epifluorescence microscope.
Western blot analysis.
Cells were lysed in Laemmli SDS loading buffer, followed by boiling for 5 min. An equal protein amount was loaded on SDS-PAGE, and proteins were electrotransferred onto nitrocellulose membranes. The membranes were washed in Tris-buffered saline with 0.1% Tween, blocked in 5% milk, and incubated with the appropriate primary antibodies at 4°C overnight, followed by incubation with horseradish peroxidase-labeled secondary antibodies, and developed by enhanced chemiluminescence.
Sucrose fractionation of low-density Triton X-100 insoluble membrane domains.
Low-density caveolin-enriched membrane fractions were isolated similar to previously described (47) with minor modifications. Cells were lysed with 0.1% Triton X-100 in 3-(N-morpholino)propanesulfonic acid-buffered saline (MBS) containing proteases inhibitors. Homogenization was carried out with 10 strokes of a loosely fitting Dounce homogenizer. A 2-ml amount of the homogenate was adjusted to 45% sucrose and 0.05% TX-100 by the addition of 2 ml of 90% sucrose prepared in MBS, placed at the bottom of an ultracentrifuge tube, overlaid with 5 ml of 35% sucrose, topped with 2 ml of 15% sucrose (in MBS without TX-100), and centrifuged at 35,000 rpm for 20 h in an SW41 rotor (Beckman Instruments). A light scattering band confined to the 35–15% sucrose region was observed that contained flotilin-1 but excluded most other cellular proteins such as heat shock protein 90 (Hsp90). Gradient fractions (1 ml) were collected from the top of the tube to yield a total of 11 fractions. A portion of fraction 2, which contains the light-scattering band, was diluted five times with MBS and pelleted by centrifugation at 39,000 rpm for 1 h to yield concentrated fraction 2*. Fifteen microliters of fraction 2* and selected fractions 2, 7, and 11 were separated by SDS-PAGE, and the gel was either stained with Coomassie Simple Blue (CB, Invitrogen) to determine protein distribution between each fraction or protein was transferred to nitrocellulose and Western blotted with antibodies against Cav-2, Cav-1, Flo-1, and Hsp-90.
Detergent-free sucrose fractionation of lipid raft and caveolar domains.
Cells were scraped into 500-mm sodium carbonate (pH 11) containing proteases and phosphatases inhibitors and sonicated (three 20-s bursts at 30% of maximal power). The homogenate (0.4 ml) was then adjusted to 45% of sucrose by the addition of 0.4 ml of 90% sucrose prepared in MBS (25 mM MES, pH 6.5, 0.15 mM NaCl) and placed at the bottom of an ultracentrifuge tube, overlaid with 3.2 ml of 35% and topped with 0.8 ml of 15% sucrose (total volume of 4.8 ml), and centrifuged at 44,000 rpm for 18 h in an MLS50 rotor (Beckman Instruments, Palo Alto, CA). Fractions of 12 × 0.4 ml were collected from the top and subjected to SDS-PAGE, followed by immunoblotting. For simplicity, the top three fractions containing Cav-1/2 and Flo-1 were pooled and concentrated, yielding light (L) lipid raft and caveolar membranes. Equal protein amount of light membranes was then analyzed side by side with the pooled bottom three heavy (H) fractions by SDS-PAGE, followed by Coomassie blue (CB) to confirm protein content and distribution as well as by Western blot to determine relative distribution of indicated TβRs and Smad2/3 between L and H analysis shown in Fig. 9.
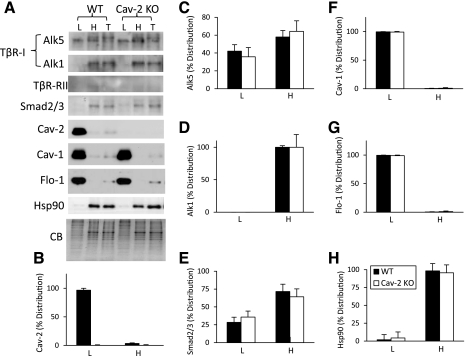
Fig. 9.
Detergent-free sucrose fractionation of TGF-β receptors and Smad2/3 in Cav-2-negative and -positive MLECs. WT and Cav-2 KO MLECs cells were lysed with sodium carbonate (pH 11) and sonicated, followed by a sucrose floatation gradient as described in materials and methods. Equal amounts of protein were loaded for pooled and concentrated top 3 light (L) fractions, bottom pooled 3 heavy (H) fractions and compared with total (T) cell lysates. A: immunoblotting with indicated antibodies and staining with Coomassie blue (CB) (compressed by 3-fold in the vertical dimension). B–H: relative (%) distribution of densitometric signal values expressed as means ± SD (n = 3) for given proteins normalized against CB between L and H.
The densitometric assessment of protein expression or phosphorylation levels.
The densitometric values for indicated proteins were determined using Image J (NIH). The data are expressed as densitometric ratios of the expression or phosphorylation levels of specific proteins to Hsp90 or to total expression level of a given protein of which phosphorylation level was assessed from one representative out of three total experiments. Alternatively, the data for sucrose fractionation assays (Fig. 2B and Fig. 9) are presented as percent distribution of total densitometric signal between each selected fraction.
RNA isolation and quantification of specific gene expression by real-time PCR.
Total RNA isolation from proliferating MLECs was performed with TRI reagent (Sigma). Isolated RNA of 1 μg was then reverse transcribed into cDNA using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). Relative expression levels of PAI-1, Col 1, and fibronectin were determined by real-time PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene, and gene expression was measured using QuantiTect SYBR Green RT-PCR kit (Qiagen) with the Bio-Rad iQ5 Optical Module plus BIO-RAD iCycler Thermal Cycler.
The following primers were used for the amplification of mouse. PAI-1 (RefSeq ID: NM_008871): forward, 5′-GCCTCCTCATCCTGCCTAA-3′ and reverse, 5′-GCCAGGGTTGCACTAAACAT-3′; Col 1 (RefSeq ID: NM_007742): forward, 5′-ACATGTTCAGCTTTGTGGACC-3′ and reverse, 5′-TAGGCCATTGTGTATGCAGC-3′; fibronectin (RefSeq ID: NM_010233): forward, 5′-GGAGTGGCACTGTCAACCTC-3′ and reverse, 5′-ACTGGATGGGGTGGGAAT-3′; GAPDH (RefSeq ID: NM_008084): forward, 5′-CGTCCCGTAGACAAAATGGT-3′ and reverse, 5′-TTGATGGCAACAATCTCCAC-3′. Thermal conditions were 15 min at 95°C, 40 cycles of 10 s at 95°C, 30 s at 52°C, and 30 s at 72°C. Values are expressed as fold induction by TGF-β calculated based on the amount of target mRNA normalized to the endogenous reference GAPDH mRNA based on the following equation: 2−ΔΔCt.
Statistical analysis.
The data are expressed as means ± SD, and where indicated were analyzed using one-way ANOVA with Tukey's posttest analysis for comparison of intra- as well as intergroup variance.
RESULTS
Cav-2 KO MLECs are more susceptible to TGF-β-induced inhibition of cell proliferation relative to their WT counterparts.
Since TGF-β is a known inhibitor of cell proliferation including many EC types, in the current study, we examined potential differences in anti-proliferative responses between MLECs isolated from WT and Cav-2 KO C57Bl6 mice. These cells were previously shown to express such major EC-specific marker proteins as Flk-1, PECAM-1, VE-cadherin, and endothelial nitric oxide synthase (eNOS) but not α-actin (a marker of mural cells, such as vascular smooth muscle cells and pericytes) (55).
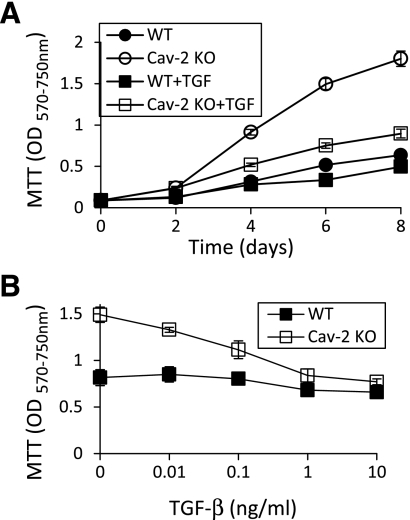
As shown in Fig. 1, using MTT colorimetric proliferation assay, we have determined that TGF-β is a more robust anti-proliferative cytokine in Cav-2 KO relative to WT MLECs. Time-dependent treatment with TGF-β (Fig. 1A) revealed that after 4 days of incubation, there was only marginal inhibitory effect of TGF-β (1 ng/ml) on WT MLECs proliferation (OD values of 0.32 ± 0.01 vs. 0.28 ± 0.04 for control and TGF-β treated cells, respectively). In contrast to WT, treatment with TGF-β resulted in an appreciable anti-proliferative effect in Cav-2 KO MLECs (OD values of 0.91 ± 0.03 vs. 0.52 ± 0.02 for control and TGF-β treated cells, respectively) (Fig. 1A).
Fig. 1.
Increased susceptibility of caveolin-2 (Cav-2) knockout (KO) mouse lung endothelial cells (MLECs) to transforming growth factor-β (TGF-β)-induced inhibition of cell proliferation. Wild-type (WT) and Cav-2 KO MLECs were plated at 4 × 103 cells per well of 24-well plate and incubated without or with TGF-β. At indicated time points, cells were processed for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) proliferation assay and optical density (OD) was measured using a plate reader with a test wavelength of 570 nm and a reference wavelength of 750 nm (570–750 nm) to obtain a sample signal and expressed as means ± SD of replicate samples (n = 3). Values are from one representative out of a total 4 experiments. A: time-dependent effect of TGF-β (1 ng/ml) on MLEC proliferation up to 8 days. B: concentration-dependent effect of TGF-β (0.01–10 ng/ml) on MLECs proliferation determined after 6 days of treatment.
Next, we have determined a concentration-dependent effect of TGF-β. Specifically, we have compared proliferation in WT and Cav-2 KO cells in the absence or presence of TGF-β (0.01–10 ng/ml) for 6 days (Fig. 1B).The OD values reached 0.82 ± 0.09, 0.85 ± 0.08, 0.80 ± 0.01, 0.68 ± 0.05, and 0.66 ± 0.05 for control WT cells and cells treated with 0.01, 0.1, 1, and 10 ng/ml TGF-β, respectively. Conversely, the OD values averaged at 1.49 ± 0.08, 1.33 ± 0.03, 1.11 ± 0.1, 0.84 ± 0.07, and 0.77 ± 0.03 for control Cav-2 KO cells and cells treated with 0.01, 0.1, 1, and 10 ng/ml TGF-β, respectively (Fig. 1B).
Retrovirally expressed human Cav-2 in Cav-2 KO MLECs shows correct targeting to plasma membrane lipid raft/caveolar microdomains and does not affect expression levels and subcellular targeting of endogenous Cav-1.
We have used retroviral expression approach to express human Cav-2 (Cav-2-pBABE) in Cav-2 KO MLECs. As seen in Fig. 2A, immunoblotting with a specific antibody that recognizes both mouse and human Cav-2 revealed significant expression of human Cav-2 (Fig. 2A; top immunoblot). Importantly, reexpressed Cav-2 did not affect the expression levels of endogenous Cav-1 in Cav-2 KO MLECs (Fig. 2A; top, second immunoblot and first bottom graph; Cav-2-pBABE vs. empty vector pBABE). We have also performed immunoblotting with antibodies against TβR-Is: Alk1 and Alk5 as well as TβR-II and did not observe significant differences between Cav-2-positive and -negative MLECs (top, 3rd-5th immunoblot and 2nd-4th graph), suggesting that Cav-2 expression does not affect the expression levels of the aforementioned receptors.
Next, we have also determined targeting to lipid raft/caveolar membranes of retrovirally expressed Cav-2 using sucrose gradient fractionation of low-density Triton X-100 insoluble membrane domains. Retrovirally expressed Cav-2 (Fig. 2B; top right immunoblot and top right bottom graph) cofractionates with Cav-1 (top, 2nd immunoblot and graph from the top) and a marker of lipid raft/caveolar membranes, Flo-1 (3rd immunoblot and graph from the top) in top fraction 2 of sucrose gradient but not with a cytosolic/heavy membrane marker Hsp-90 (Fig. 2B; bottom immunoblot and 2nd to last graph; fraction 11). Furthermore, retrovirally expressed Cav-2 did not visibly affect the relative distribution of endogenous Cav-1 between selected fractions, since Cav-1 cofractionated with Flo-1 in top fraction 2 but not with Hsp90 in bottom fraction 11 in both pBABE and Cav-2-pBABE cells (Fig. 2B; compare left vs. right 2nd immunoblot from the top and corresponding graphs).
Monoclonal antibody to Cav-2 (Clone 65; BD) used for immunoblotting (Fig. 2, A and B) as well as polyclonal antibody (G15; Santa Cruz) performed very poorly for immunofluorescence labeling in MLECs (not shown). Therefore, to determine the efficiency (% cells expressing Cav-2) and subcellular targeting of human Cav-2 reexpressed using retroviral approach, we took advantage of and used a rabbit polyclonal antibody against human serine 23-phosphorylated Cav-2 (P-Ser 23-Cav-2) previously generated in our laboratory that had been well characterized for immunofluorescence staining (47). While immunofluorescence labeling with this antibody revealed no P-Ser 23-Cav-2-specific staining in Cav-2 KO cells infected with empty vector (Fig. 2C; top left panel labeled as “pBABE”), nearly all Cav-2 KO cells infected with retroviral vector expressing human Cav-2 (Fig. 2C; top middle panel labeled as “Cav-2-pBABE”) were positively stained with P-Ser 23-Cav-2-specific red fluorescence. Importantly, P-Ser 23-Cav-2-specific staining was primarily localized to the plasma membrane (arrows) (Fig. 2C; top right panel labeled “Cav-2-pBABE (4× zoom),” consistent with previously described subcellular location pattern observed for Cav-2 and P-Ser 23-Cav-2 (46, 47). Furthermore, immunofluorescence staining with rabbit polyclonal antibody against Cav-1 revealed that Cav-2 expression did not affect Cav-1 subcellular localization, which was primarily confined to the plasma membrane in both control and Cav-2-expressing cells (depicted by arrows in Fig. 2C; bottom left vs. right for pBABE and Cav-2-pBABE cells, respectively).
Thus both our immunoblotting analysis of the sucrose gradient fractions and the immunofluorescence data suggest that the human Cav-2 delivered using retroviral approach targets primarily to plasma membrane lipid raft/caveolar membranes. Furthermore, unlike in many overexpression approaches, the levels of retrovirally expressed human Cav-2 are not higher than the levels of endogenous Cav-2 observed in WT MLECs. Importantly, the retrovirally expressed Cav-2 does not affect the levels of Cav-1 expression or subcellular targeting to plasma membrane lipid raft and caveolar microdomains. Thus any change that we might see in the responses to TGF-β in the Cav-2-reexpressing cells would be attributable to a direct, physiological effect of Cav-2 and not due to either the restoration of WT Cav-1 level or other nonspecific effects related to Cav-2 overexpression. Therefore, to avoid concerns that WT MLEC expressed more Cav-1 than Cav-2 KO MLEC, in addition to WT MLECs, we have also used pBABE- and Cav-2-pBABE-infected MLECs, expressing equal levels of Cav-1 that are correctly targeted to plasma membrane lipid raft and caveolar microdomains.
Reexpressed Cav-2 in Cav-2 KO MLECs inhibits anti-proliferative effect of TGF-β.
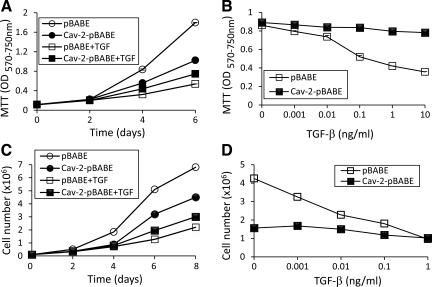
First, we compared the anti-proliferative responses in the newly characterized pBABE and Cav-2-pBABE cells using MTT colorimetric proliferation assay. Specifically, pBABE and Cav-2-pBABE cells were treated with TGF-β at 1 ng/ml for up to 6 days (Fig. 3A). As previously reported for Cav-2 KO cells (Fig. 1), proliferation of pBABE-expressing cells was robustly inhibited by TGF-β in a time-dependent manner. Importantly, Cav-2 reexpression in Cav-2 pBABE cells substantially reduced the inhibitory effect of TGF-β. Specifically, after 4 days of incubation, treatment with TGF-β resulted in an appreciable anti-proliferative effect in pBABE MLECs (OD values of 0.84 ± 0.014 vs. 0.32 ± 0.01 for control and TGF-β-treated cells, respectively) (Fig. 3A; open circles and squares). In contrast, there was only marginal inhibitory effect of TGF-β (1 ng/ml) on the proliferation of Cav-2 pBABE (OD values of 0.56 ± 0.02 vs. 0.44 ± 0.01 for control and TGF-β-treated cells, respectively) (Fig. 3A; solid circles and squares).
Fig. 3.
Reduced susceptibility of MLECs reexpressing Cav-2 to the time and concentration-dependent anti-proliferative effect of TGF-β. A and B: pBABE and Cav-2-pBABE MLECs were plated at 4 × 103 cells per well of 24-well plate and incubated without or with TGF-β, followed by processing for MTT proliferation assay. OD was measured using a plate reader with a test wavelength of 570 nm and a reference wavelength of 750 nm (570–750 nm) to obtain a sample signal and expressed as means ± SD of replicate samples (n = 3). Values are from one representative out of a total four experiments. A: time-dependent effect of TGF-β (1 ng/ml) on MLEC proliferation up to 6 days. B: concentration-dependent effect of TGF-β (0.001–10 ng/ml) on MLECs proliferation determined after 5 days of treatment. C and D: pBABE and Cav-2-pBABE MLECs were plated at 105 cells per 100-mm dish, cell numbers were determined at the indicated time points using Cell Countess. Values are from one representative out of a total four experiments. C: time-dependent effect of TGF-β (1 ng/ml) on MLEC proliferation up to 8 days. D: concentration-dependent effect of TGF-β (0.001–1 ng/ml) on MLECs proliferation determined after 5 days of treatment.
Next, we have determined the concentration-dependent effect of TGF-β on the proliferation of pBABE and Cav-2-pBABE cell with MTT assay. Specifically, pBABE and Cav-2-pBABE cells were grown in the absence or presence of TGF-β (0.001–10 ng/ml) for 5 days (Fig. 3B). For control pBABE cells and cells treated with 0.001, 0.01, 0.1, 1, and 10 ng/ml TGF-β, the OD values reached 0.86 ± 0.01, 0.80 ± 0.01, 0.74 ± 0.01, 0.52 ± 0.01, 0.42 ± 0.01, and 0.36 ± 0.01, respectively (Fig. 3B; open squares). Conversely, for control Cav-2-pBABE cells and cells treated with 0.01, 0.1, 1, and 10 ng/ml TGF-β, the OD values were 0.89 ± 0.01, 0.87 ± 0.01, 0.84 ± 0.01, 0.84 ± 0.01, 0.80 ± 0.01, and 0.78 ± 0.01 (Fig. 3B; filled squares).
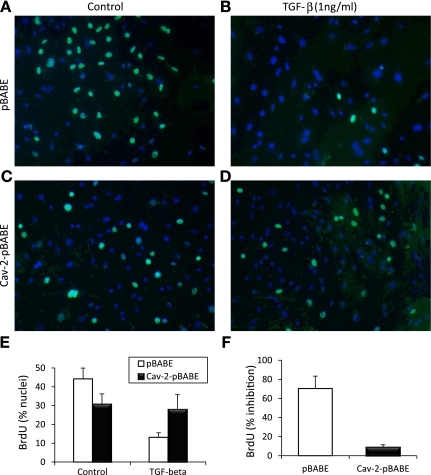
We next used cell count as an independent proliferation assay to directly assess the anti-proliferative effect of TGF-β in pBABE and Cav-2-pBABE cells. Specifically, we treated pBABE and Cav-2-pBABE cells with TGF-β at 1 ng/ml on proliferation for up to 8 days (Fig. 3C). Treatment with TGF-β robustly inhibited cell proliferation of pBABE cells in a time-dependent manner and Cav-2 reexpression substantially reduced the inhibitory effect of TGF-β. Specifically, after 6 days of incubation, treatment with TGF-β resulted in a profound anti-proliferative effect in pBABE cells (cell number values of 5.1 × 106 vs. 1.3 × 106 for control and TGF-β-treated cells, respectively) (Fig. 3C; open circles and squares). In contrast, only a modest inhibitory effect of TGF-β was observed on Cav-2 pBABE proliferation (cell number values of 3.2 × 106 vs. 2 × 106 for control and TGF-β-treated cells, respectively) (Fig. 3C; filled circles and squares). To determine how reexpressed Cav-2 affects concentration-dependent anti-proliferative effect of TGF-β on MLECs, we have compared cell proliferation in pBABE and Cav-2-pBABE cells in the absence or presence of TGF-β (0.001–1 ng/ml) for 5 days (Fig. 3, D–F). For control pBABE cells and cells treated with 0.001, 0.01, 0.1, and 1 ng/ml TGF-β, the cell count values reached 4.25, 3.25, 2.28, 1.8, and 0.96 × 106 cells (Fig. 3D; open squares). Conversely, for control Cav-2-pBABE cells and cells treated with 0.001, 0.01, 0.1, and 1 ng/ml of TGF-β, the cell count values approached 1.56, 1.68, 1.5, 1.19, and 1.02 × 106 cells per 100-mm dish (Fig. 4D; filled squares). To determine how TGF-β affects cell cycle progression into S phase, we have used BrdU incorporation assay followed by fluorescent microscopy to detect S phase-associated (DNA synthesizing) cells (Fig. 5, A–C). Treatment of pBABE cells with TGF-β (1 ng/ml) for 4 days resulted in a robust inhibition in the number of BrdU-positive cells (Fig. 4; see green-labeled nuclei in B vs. A). In contrast, TGF-β had very little effect on fraction of BrdU-positive Cav-2-pBABE cells (Fig. 4; green-labeled nuclei in D vs. C). Detailed quantitative analysis from 9 independent fields revealed 44.2 ± 5.8% for control vs. 13.1 ± 2.4% for TGF-β-treated BrdU-positive pBABE cells (Fig. 4E; open bars). Conversely, there was 30.8 ± 5.5% control vs. 28.0 ± 7.8% TGF-β-treated BrdU-positive Cav-2-pBABE cells (Fig. 4E; filled bars). The calculated percent inhibitory effect of TGF-β was 70.3% in pBABE and only 8.9% in Cav-2-pBABE cells (Fig. 4F). Overall, BrdU incorporation data correlates well with cell proliferation data assessed by MTT and cell count (Fig. 3) proliferation assays and further support the inhibitory role of Cav-2 in regulating the anti-proliferative function of TGF-β in ECs.
Fig. 4.
Reduced sensitivity of Cav-2 reexpressing MLECs to TGF-β-mediated inhibition of bromodeoxyuridine (BrdU) incorporation. pBABE or Cav-2-pBABE MLECs were plated at 5 × 103 per chamber of an 8-chamber glass slide precoated with 0.2% gelatin. One day later, cells were left untreated (A and C, for pBABE and Cav-2-pBABE cells, respectively) or treated with 1 ng/ml of TGF-β (B and D) for 4 days, followed by pulsing with BrdU for 2 h, fixing, and immunofluorescence staining with fluorescein isothiocyanate-conjugated anti-BrdU antibody (green) and DAPI (blue). Note the dramatic decreases in frequency of BrdU-positive (green) cells in pBABE treated with TGF-β (B) vs. control cell (A), while lack of an obvious inhibition in Cav-2-pBABE cells (C vs. D). E: percent BrdU-positive cells was calculated and expressed as means ± SD from independent microscopic fields (n = 9). Values are from one representative out of a total three experiments. F: percent inhibition by TGF-β = (100% − % nuclei in TGF-β-treated samples)/% nuclei in respective nontreated (Control) samples × 100%.
Fig. 5.
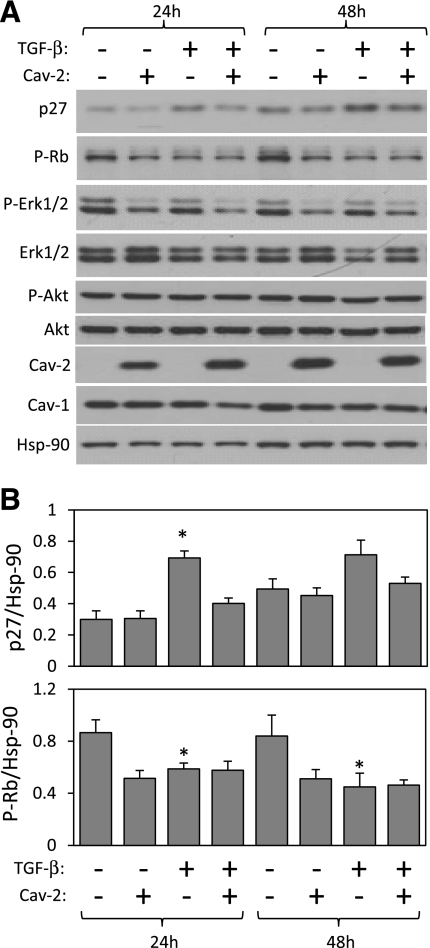
Effect of TGF-β on the expression or phosphorylation levels of selected inhibitors and activators of cell proliferation in Cav-2-negative and -positive MLECs. pBABE and Cav-2-pBABE MLECs were plated at low density (3 × 105 per 150-mm dish), followed by incubation with complete medium without or with TGF-β (1 ng/ml) for 24 and 48 h. At indicated time points, cells were lysed and SDS-PAGE resolved protein lysates immunoblotted with the indicated antibodies to p27, phosporylated Rb protein (P-Rb), phosphorylated ERK1/2 (P-ERK 1/2), total ERK 1/2, phosphorylated Akt (P-Akt), total Akt, caveolins, and Hsp-90. Note that both 24 and 48 h treatment with TGF-β results in a greater increase in the expression levels of p27 in pBABE relative to Cav-2-pBABE cells (top panel and top graph). Also, note that 48 h treatment with TGF-β visibly reduces the P-Rb levels in pBABE but not Cav-2-pBABE (2nd immunoblot from the top and bottom graph). Finally, note that TGF-β does not affect P-ERK 1/2 nor P-Akt levels. B: densitometric ratios of p27 and P-Rb to Hsp90 quantified using Image J, which were selectively affected by TGF-β treatment in pBABE relative to Cav-2-pBABE cells and expressed as means ± SD (n = 3). *P < 0.05 vs. respective control sample; #P < 0.05 vs. respective TGF-β-treated sample at given time point.
Cav-2 reduces the regulatory effect of TGF-β on the expression levels of p27 and inactivating phosphorylation levels of pRb cell cycle inhibitors in MLECs.
To gain further insight as to how Cav-2 controls TGF-β regulatory effect on cell cycle progression in MLECs, we performed immunoblotting with antibodies against total levels of the cdk inhibitor p27Kip1, the phosphorylated (inactive) form of the Rb protein as well as phosphorylated and total levels of ERK1/2 and Akt in control and TGF-β (1 ng/ml)-treated MLECs (Fig. 5). A 24-h treatment with TGF-β resulted in a statistically significant increase in p27 expression levels in pBABE but not in Cav-2-pBABE MLECs (Fig. 5A; top immunoblot and Fig. 5B; top). Consistent with Cav-2-pBABE, but in contrast to pBABE-MLECs, 24-h treatment with TGF-β failed to significantly upregulated p27 expression in WT MLECs (Fig. 6B, top immunoblot and graph; 1st-6th lane). These data suggest that Cav-2 negatively affects the ability of TGF-β to increase p27 expression levels in ECs.
Fig. 6.
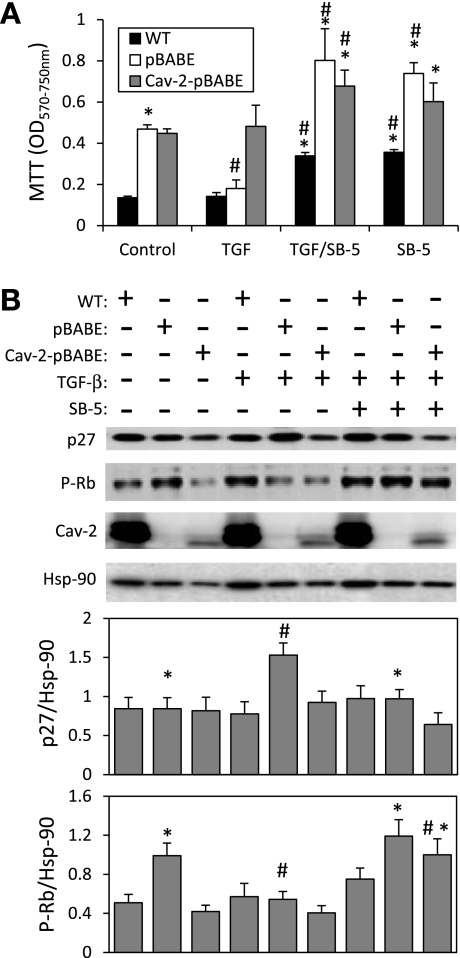
Alk5 dependence of the anti-proliferative effect of TGF-β in MLECs. A: WT MLECs and Cav-2 KO MLECs expressing pBABE or Cav-2-pBABE were plated at 4 × 103 cells per well of 24-well plate and incubated without or with TGF-β (1 ng/ml and/or SB-5 (1 μM) for 6 days, followed by processing for MTT proliferation assay and measurement of OD as described in materials and methods. The data are expressed as means ± SD of replicate samples (n = 3). *P < 0.05 vs. respective TGF-β-treated sample; #P < 0.05 vs. respective control sample. B: WT MLECs and Cav-2 KO MLECs expressing pBABE or Cav-2-pBABE were plated at 3 × 105 per 150-mm dish and incubated without or with TGF-β (1 ng/ml) and/or SB-5 (1 μM) for 24 h, followed by lyses and immunoblotting with the indicated antibodies. Graphs, densitometric ratios of p27 (top) and P-Rb (bottom) to Hsp90 quantified using Image J and expressed as means ± SD (n = 3). *P < 0.05 vs. respective TGF-β-treated sample; #P < 0.05 vs. respective control sample.
In addition, 24 and 48 h treatment with TGF-β resulted in a statistically significant reduction of the “inactivating” phosphorylation level of the Rb protein in pBABE but not in Cav-2-pBABE MLECs (Fig. 5A; 2nd panel from the top; P-Rb and Fig. 5B, bottom). Consistent with Cav-2-pBABE, but in contrast to pBABE-MLECs, 24 h treatment with TGF-β failed to significantly downregulate the levels of phosphorylated Rb protein in WT MLECs (Fig. 6B, 2nd immunoblot and bottom graph, 1st-6th lane). These data suggests that Cav-2 negatively regulates TGF-β-induced activation (reduced phosphorylation) of Rb protein in ECs.
In contrast to p27 and pRb, TGF-β treatment did not affect the phosphorylation levels of ERK1/2 and Akt (Fig. 5A; 3rd-6th immunoblot from the top), suggesting that the anti-proliferative effect of TGF-β in MLECs is independent of ERK1/2 and Akt activation. Overall, these data suggest that Cav-2 negatively influences anti-proliferative effect of TGF-β in ECs by reducing the ability of this cytokine to upregulate the expression levels of cdk inhibitor p27Kip1 and suppress the inactivating phosphorylation of the G1 to S transition inhibitor Rb.
The enhanced inhibitory effect of TGF-β on the proliferation of Cav-2-negative MLEC is TβR-I/Alk5 dependent.
Since TGF-β receptor type I Alk5 is commonly believed to be responsible for anti-proliferative function of TGF-β in various cell types, including ECs, we have used SB-505124 (SB-5), a pharmacological inhibitor of Alk5, to determine if Alk5 is indeed regulated by Cav-2 in MLECs. Treatment with SB-5 (1 μM) for 6 days not only reversed the inhibitory effect of TGF-β (1 ng/ml) in pBABE MLECs but also increased proliferation of both Cav-2-negative and -positive MLECs relative to control cells (Fig. 6A). Pretreatment with SB-5 also reversed TGF-β-induced upregulation of p27 and downregulation of pRB phosphorylation in Cav-2-negative pBABE MLECs (Fig. 6B; 1st-2nd immunoblot from the top and bottom graphs; 8th vs. 5th lane). Consistent with proliferation data, there was a trend for SB-5 to increase phosphorylation of Rb protein in Cav-2-positive and -negative MLECs relative to their respective controls (Fig. 6B; 2nd immunoblot and bottom graph; 7th-9th vs. 1st-3rd lane), Interestingly, no significant downregulation of p27 in hyperproliferating SB-5-treated MLECs was observed relative to their respective control cells (Fig. 6B; top immunoblot and graph, 7th-9th vs 1st-3rd lane). Overall, the full reversal of hyperinhibitory effect of TGF-β on proliferation of pBABE cells by SB-5 suggests that ALK5 mediates the anti-proliferative effect of TGF-β and that Cav-2 may negatively regulate TGF-β-stimulated Alk5 activity in ECs. Furthermore, the stimulatory effect of SB-5 on both pBABE and Cav-2-pBABE MLEC proliferation in the absence of TGF-β suggests that there is some basal anti-proliferative activity of Alk5 in these cells, which does not appear to be regulated by Cav-2.
Cav-2 negatively regulates TGF-β-induced phosphorylation of Smad2 and 3 in MLECs and primary human ECs.
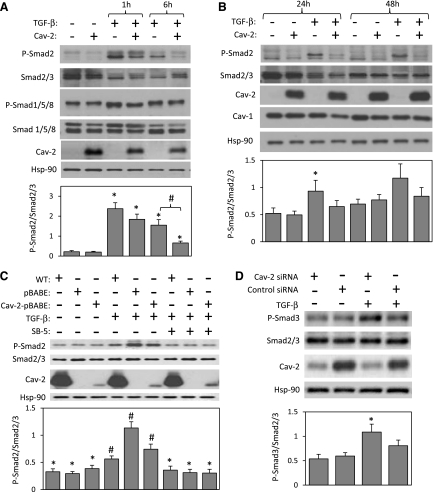
Since Smad2/3 is a key downstream mediator of TGF-β-induced anti-proliferative effect in many cell types, we have examined Smad2/3 activation by immunoblotting with phospho-Smad2 (P-Smad2)-specific antibody. As seen in Fig. 7A, top immunoblot and bottom graph, treatment with TGF-β dramatically increased Smad2 phosphorylation at the 1-h time point. The TGF-β-induced Smad2 phosphorylation remained high in pBABE but was much reduced in Cav-2-pBABE cells at the 6-h time point. On average, P-Smad2-to-Smad2 ratio increased by 10.9-fold in pBABE and by 9.2-fold in Cav-2-pBABE cells following 1 h treatment with TGF-β; and by 7.1-fold in pBABE versus 3.3-fold in Cav-2-pBABE cells after 6 h treatment with TGF-β (Fig. 7A; 1st and 2nd immunoblot from the top and bottom graph). Consistent with Cav-2-pBABE but in contrast to pBABE-MLECs, 6 h treatment with TGF-β resulted in a modest increase in Smad2 phosphorylation in WT MLECs (Fig. 7C; top 1st-2nd immunoblot and bottom graph; 4th-6th vs. 1st-3rd lane). Because TGF-β could possibly affect Alk-1 in ECs, we have also examined a downstream phosphorylation target for the latter kinase, i.e., Smad1/5/8. Unlike P-Smad2, up to 6 h treatment with TGF-β did not have a statistically significant effect on Smad1/5/8 phosphorylation in both pBABE and Cav-2-pBABE MLECs (Fig. 7A; 3rd-4th immunoblot), suggesting that Alk1-Smad1/5/8 pathway is not appreciably activated in these cells by TGF-β. We have further explored activation of Alk5-Smad2 pathway in MLECs treated with TGF-β for 24 and 48 h. Interestingly, increased Smad2 phosphorylation was still statistically significant in pBABE but not in Cav-2-pBABE cells treated with TGF-β for 24 h (Fig. 7B; 1st-2nd immunoblot and bottom graph). To confirm that the increase in Smad2 phosphorylation is Alk5 dependent, WT, pBABE, and Cav-2-pBABE MLECs were pretreated with SB-5 (1 μM) for 1 h before treatment with TGF-β for 6 h (Fig. 7C). Consistent with its effect on proliferation (shown in Fig. 6), SB-5 also reversed stimulatory effect of TGF-β on Smad2 phosphorylation in all cell types, suggesting involvement of Alk5 (Fig. 7C; top 1st-2nd immunoblot and bottom graph; 7th-9th vs. 4th-6th lane). To determine whether Cav-2 could possibly affect Smad2 phosphorylation in human primary ECs, we have treated HUVEC with TGF-β (5 ng/ml). Although, we were unable to detect Smad2 phosphorylation as we did in MLECs, we could see an increase in Smad3 phosphorylation. Specifically, treatment with TGF-β for 3 h resulted in a statistically significant increase in Smad3 phosphorylation in HUVECs transfected with Cav-2 but not in cells treated with control siRNA (Fig. 7D). These data suggest that Cav-2 may negatively regulate TGF-β-induced signaling and function in primary human ECs. Overall, our data suggests that Cav-2 may play an important role in reducing TGF-β signaling in ECs.
Fig. 7.
Effect of TGF-β on Smad phosphorylation in Cav-2-negative and -positive MLECs and human umbilical vein endothelial cells (HUVECs) with small interfering RNA (siRNA)-induced knockdown of Cav-2. A and B: pBABE and Cav-2-pBABE MLECs were plated at low density (3 × 105 per 150-mm dish), followed by incubation with complete medium without or with TGF-β (1 ng/ml) for up to 48 h. At indicated time points, cells were lysed and SDS-PAGE resolved protein lysates immunoblotted with the indicated antibodies to phosphorylated Smad2 (P-Smad2), total Smad2, phosphorylated Smad1/5/8 (P-Smad1/5/8), total Smad1/5/8, Cav-2, Cav-1, and Hsp-90. Graphs, densitometric ratios of P-Smad2/Smad2/3 from control and treated with TGF-β for 1 and 6 h (A) or for 24 and 48 h (B) samples quantified based on the above immunoblots and expressed as means ± SD (n = 3). *P < 0.05 vs. respective control sample; #P < 0.05 for vs. respective TGF-β-treated sample at given time point. C: WT MLECs and Cav-2 KO MLECs expressing pBABE or Cav-2-pBABE were plated at 3 × 105 per 150-mm dish and incubated without or with TGF-β (1 ng/ml) and/or SB-5 (1 μM) for 6 h, followed by lyses and immunoblotting with the indicated antibodies. Graph in the bottom represents densitometric ratios of P-Smad2/Smad2/3 quantified based on the above immunoblots and expressed as means ± SD (n = 3). *P < 0.05 vs. respective TGF-β-treated sample; #P < 0.05 vs. respective control sample. D: HUVEC transfected with Cav-2 and control siRNA were treated without or with TGF-β (5 ng/ml) for 3 h, followed by lyses and immunoblotting with indicated antibodies. Graph in the bottom represents densitometric ratios of P-Smad3/Smad2/3 quantified based on the above immunoblots and expressed as means ± SD (n = 3). *P < 0.05 vs. respective control sample.
Cav-2 negatively regulates transcriptional activation of downstream targets of Alk5-Smad2/3 pathway.
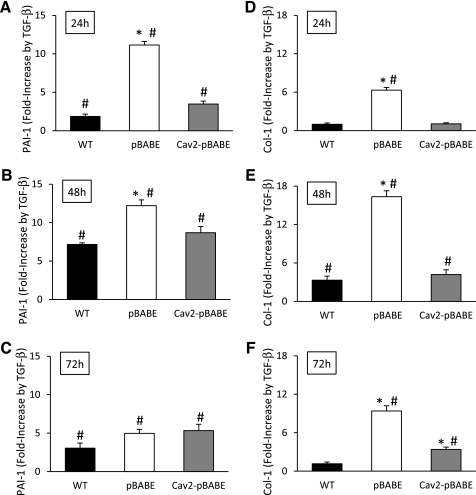
Since TGF-β is believed to inhibit EC proliferation through Alk5-Smad2/3 pathway inducing PAI-1 and deposition of extracellular matrix components such as Col 1 (2), we have performed real-time PCR to determine the expression of the above-mentioned genes (Fig. 8). Treatment with TGF-β (1 ng/ml) resulted in more rapid and profound induction of mRNA for both PAI-1 (Fig. 8, A–C) and Col 1 (Fig. 8, D–F) in pBABE relative to WT and Cav-2-pBABE cells. Specifically, for PAI-1, the fold induction by TGF-β treatment for 24, 48, and 72 h over the respective control levels was 1.9, 7.2, 3.1; 11.2, 12.2, 5.0; and 3.5, 8.7, 5.3 in WT, pBABE, and Cav-2-pBABE cells, respectively (Fig. 8, A–C). Also, for Col 1, the fold induction by TGF-β treatment for 24, 48, and 72 h over the respective control levels was 1, 3.3, 1.1; 6.3, 16.3, 9.4; and 1.1, 4.2, 3.4 in WT, pBABE, and Cav-2-pBABE cells, respectively (Fig. 8, D–F). There was no appreciable induction of fibronectin mRNA in Cav-2-negative nor -positive MLECs by TGF-β (not shown). These data suggest that Cav-2 may limit transcriptional activation of downstream targets of TGF-β Alk5-Smad2/3 pathway in ECs.
Fig. 8.
Effect of TGF-β on activin receptor-like kinase-5 (Alk5)-Smad2/3 target gene plasminogen activator inhibitor-1 (PAI-1), and collagen type-1 (Col-1) expression in Cav-2-negative and -positive MLECs. WT MLECs and Cav-2 KO MLECs expressing pBABE or Cav-2-pBABE were plated at low density (3 × 105 per 150-mm dish), followed by incubation with complete medium without or with TGF-β (1 ng/ml) for up to 72 h. At indicated time points, total RNA was isolated and real-time PCR performed as described in materials and methods. Data are expressed as fold induction by TGF-β calculated based on the relative amount of target mRNA normalized to the endogenous reference GAPDH mRNA and represented as means ± SD of 3 replications done in duplicates. *P < 0.05 vs. respective WT sample; #P < 0.05 vs. respective control (minus TGF-β) sample at given time point.
Cav-2 does not significantly alter the targeting of Alk5 and Smad2/3 to caveolar/lipid membranes.
To determine whether Cav-2 could regulate targeting of TβRs to caveolar and lipid raft microdomains, we have subjected both WT and Cav-2 KO MLECs to detergent-free sucrose floatation gradient (Fig. 9). Interestingly, loss of Cav-2, which predominantly targets to light fractions of sucrose gradient (Fig. 9A; 5th immunoblot from the top and Fig. 9B) did not affect the relative distribution of Alk5, Alk1, and Smad2/3 between light and heavy fractions of sucrose gradient (Fig. 9A, immunoblots 1, 2, 4; and 9, C–E). The possible effect of Cav-2 on TβRII remains inconclusive due to very low levels detected by TβRII antibody, which did not allow for a reliable densitometric quantitation. Overall, this sucrose floatation gradient data suggests that Cav-2 does not seem to regulate targeting of Alk5 and Smad2/3 to lipid raft and caveolar microdomains.
DISCUSSION
In the current study using pure populations of ECs isolated from lungs of WT and Cav-2 KO mice combined with retroviral reexpression of Cav-2 in Cav-2 KO ECs, we provide for the first time evidence for the negative role of Cav-2 in regulating TGF-β signaling and function. Specifically, we show that Cav-2-negative MLECs are more susceptible to anti-proliferative effect of TGF-β than WT counterparts and Cav-2 KO MLEC with retrovirally reexpressed Cav-2.
Interestingly, the levels of retrovirally reexpressed Cav-2 in Cav-2 KO MLECs are insufficient to significantly increase the levels of endogenous Cav-1 and do not affect Cav-1 targeting to caveolar and lipid raft microdomains of plasma membrane as determined by gradient fractionation and fluorescence microscopy immunolocalization data. Therefore, the functional effects of reexpressed Cav-2 can be interpreted as direct, i.e., independent of Cav-1 expression levels as well as subcellular targeting. Thus although Cav-1 has been previously shown to regulate TGF-β action in various cell types (15, 18, 24, 40, 41, 52), our data suggests that Cav-2 might be able to affect TGF-β signaling and function independently of Cav-1.
The reduced anti-proliferative effect of TGF-β in WT and Cav-2 reexpressing ECs could be observed with three independent proliferation assays: MTT, cell count, and BrdU incorporation. The relatively low inhibitory effect of TGF-β on WT MLEC proliferation is consistent with the fact that, although TGF-β is a potent inhibitor of proliferation in epithelial cells, WT ECs often display poor responses to TGF-β (12, 14, 29). In contrast, a substantial anti-proliferative effect of TGF-β could be seen in the Cav-2 KO cells at suboptimal concentrations of the cytokine (0.01–0.1 ng/ml), which had very little, if any, inhibitory effect on the proliferation of WT or Cav-2 reexpressing ECs. The profound reduction of inhibitory effect of TGF-β on the fraction of S phase-associated MLECs reexpressing Cav-2 as determined by BrdU incorporation assay, suggests that ECs lacking Cav-2 are more prone to TGF-β-mediated inhibition of the G1 to S phase transition.
In a variety of different cell types, TGF-β inhibits cell proliferation by its ability to induce the transcription of cyclin-dependent kinase inhibitors such as p27Kip1 (26).
p27Kip1 may negatively affect the kinase activity of multiple cdk/cyclin complexes, such as cdk2/cyclin E, cdk2/cyclin A, cdk1/cyclin A, as well as cdk1/cyclin B (1, 31, 49). Therefore, the decreased upregulation of p27Kip1 in MLECs reexpressing Cav-2 in response to TGF-β can be translated into reduced inhibition of the cdk's and lessen the inhibitory effect of the latter cytokine on both G1 to S transition and progression through S and G2/M phases of the cell cycle. The G1 to S transition requires the hyperphosphorylation/inactivation of the tumor suppressor Rb (43, 44). The Rb protein is considered as a negative regulator of the cell cycle progression (30). Phosphorylation-induced inactivation of Rb releases the transcription factor E2F, allowing it to activate transcription of genes required for DNA synthesis and cell cycle progression (50). Our data showed that 24 h treatment with TGF-β significantly reduced Rb phosphorylation in Cav-2 negative but not in WT and Cav-2 reexpressing Cav-2 KO MLECs. The failure of TGF-β to upregulate p27kip1 in these cells might have been a contributing factor.
Of particular note, we also analyzed the phosphorylation states of ERK1/2 and Akt, which could be activated through Smad-independent manner by TGF-β in certain cell types (17) and are potentially important in EC proliferation. However, our immunoblotting data with phospho-Erk1/2 and phospho-Akt antibodies determined that these pathways were not regulated by TGF-β in MLECs, consistent with previously published observation that TGF-β did not affect ERK1/2 activation in mouse embryonic ECs (37).
What could be the direct target(s) of Cav-2 that is (are) responsible for its negative effect on the anti-proliferative effect of TGF-β in MLECs? ECs have been reported to express two distinct TβR-Is, namely, TβR-I/Alk5 and ALK1. SB-5, a compound previously shown to selectively inhibit Alk5 without affecting Alk1 (13), entirely reversed hyperinhibitory effect of TGF-β in ECs lacking Cav-2. Since previous studies have shown that TGF-β/Alk5 pathway leads to inhibition of EC proliferation (10, 11), our data with SB-5 suggest that Alk5 could be a primary target through which Cav-2 negatively regulates anti-proliferative function of TGF-β in ECs. Furthermore, the role for Cav-2 in inhibiting Alk5 pathway is independently supported by our observation that TGF-β causes prolonged hyperactivation of Smad2 in Cav-2 negative, relative to WT and Cav-2 reexpressing MLECs. Also, the enhanced Smad3 phosphorylation by TGF-β in primary human ECs with siRNA-mediated reduction of Cav-2 is consistent with results obtained with MLECs. Overall, our data are consistent with previous studies showing that TGF-β-ALK5-Smad2/3 pathway leads to inhibition of cell proliferation (10, 11). In agreement with this prolonged Smad2/3 activation, the expression levels of genes downstream of TGF-β-ALK5-Smad2/3 pathway such as PAI-1 and Col-1 is also more robust and prolonged in Cav-2-negative ECs treated with TGF-β. This may suggest that the protein levels of both PAI-1 and Col-1 may be higher, although we were unable to detect them by immunoblotting with commercially available antibodies (not shown). It is believed that in ECs, the activation of Alk5-Smad2–3 pathway leads to inhibition of cell proliferation through induction of PAI-1 and extracellular matrix components such as Col-1 (2). Thus our data suggest that Cav-2 negatively affects anti-proliferative effect of TGF-β, at least partially through limiting TGF-β mediated induction of EC quiescence-associated PAI-1 and Col-1.
Does Cav-2 regulate targeting of TβRs and other signaling components of TGF-β signaling to lipid raft/caveolar membranes? Our data obtained using sucrose floatation gradient suggests that Cav-2 does not significantly influence relative distribution of Alk5, Alk1, and Smad2/3 between Cav-2-positive and -negative MLECs. Also, because of limited protein quantities in lipid raft/caveolar membrane fractions, even upon fivefold concentration and overall weak signal, we could not determine whether TβR II targeting could be modulated in MLECs by Cav-2.
Another interesting question is whether Cav-2 could possibly be part of TGF-β signaling complex or interact with Alk5 or other components of this pathway. In our preliminary experiments using coimmunoprecipitation approaches with Cav-2 and Alk5 antibodies in lysates form MLECs and HUVECs (not shown), we were unable to detect coassociation of Alk5 with Cav-2. However, due to very low levels of Alk5 detected in both cell lysates and immunoprecipitates with Alk5 antibody from Santa Cruz, it is too premature to conclude whether Cav-2 does or does not interact with Alk5.
Clearly, further subcellular fractionation, colocalization, and coimmunoprecipitation studies with antibodies against additional total and posttranslationally modified TβRs and other components of TGF-β signaling cascade will be required to more precisely determine how Cav-2 regulates TGF-β signaling in ECs.
In summary, Cav-2 negatively regulates anti-proliferative function and associated signaling of TGF-β in MLECs and Smad3 phosphorylation in primary human ECs. Mechanistically, Cav-2 appears to inhibit anti-proliferative action of TGF-β by suppressing Alk5-Smad2/3 pathway, leading to decreased TGF-β-induced transcriptional activation of genes associated with more quiescent ECs such as PAI-1 and Col-1. This less activated Alk5-Smad2/3 pathway in Cav-2-positive ECs becomes inefficient in upregulating cdk inhibitor p27Kip1 and suppressing inactivating phosphorylation of the G1 to S transition inhibitor the Rb protein, resulting in markedly reduced ability of TGF-β-Alk5-Smad2/3 to prevent G1 to S transition and ultimately inhibit cell proliferation.
It is important to reconcile this new role for Cav-2 in inhibiting TGF-β-mediated anti-proliferative effect in ECs with previously described anti-proliferative role of Cav-2 (55). Although, in both cases, Cav-2 seems to act as an inhibitor, the final outcome depends on the specific context. In our previous studies, cell proliferation was evaluated only under optimal conditions, i.e., in the absence of known growth inhibitors. Under latter conditions, Cav-2 dampened pro-proliferative effect of growth factors present in serum and endothelial cell growth supplement resulting in ca. twofold reduction of proliferation. Conversely, in the presence of TGF-β, the function of Cav-2, switches from anti- to pro-proliferative one through ability of Cav-2 to dampen the anti-proliferative effect of TGF-β via Alk5-Smad2/3 pathway. Thus it is plausible to hypothesize that Cav-2 protein may act as a molecular switch preventing excessive cell responses to both pro- and anti-proliferative signals. Further studies elucidating the detailed mechanisms responsible for inhibitory regulation of TGF-β-induced signaling and function in ECs by Cav-2 will be necessary. In particular, investigation into the role of previously identified serine and tyrosine phosphorylation of Cav-2 (25, 46, 47, 51), possible interactions of Cav-2 with Alk5 or other components of TGF-β pathway in the absence and presence of TGF-β, possibility of regulation of the expression levels, and subcellular targeting of additional receptors for TGF-β, e.g., accessory receptors such as endoglin or β-glycan by Cav-2, as well as in vivo significance of our findings are clearly warranted.
GRANTS
This work was supported by National Institutes of Health Grant 1R01HL-081860 (to G. Sowa).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
We thank Drs. Michael Lisanti and Phillippe Frank for enabling us to isolate MLECs from Cav-2 KO mice, Dr. William C. Sessa for EcoPack2 cells stably producing retrovirus expressing polyoma middle T antigen, and Dr. Robert Lim for critical reading and helpful comments regarding the manuscript.
REFERENCES
- 1. Aprelikova O, Xiong Y, Liu ET. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem 270: 18195–18197, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Bohnsack BL, Hirschi KK. Red light, green light: signals that control endothelial cell proliferation during embryonic vascular development. Cell Cycle 3: 1506–1511, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Byfield SD, Roberts AB. Lateral signaling enhances TGF-β response complexity. Trends Cell Biol 14: 107–111, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chan YG, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol 11: 629–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Das K, Lewis RY, Scherer PE, Lisanti MP. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J Biol Chem 274: 18721–18728, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-β receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol 20: 713–719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452., 2001 [DOI] [PubMed] [Google Scholar]
- 8. Fujimoto T, Kogo H, Nomura R, Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci 113: 3509–3517, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med 13: 301–307, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol Cell 12: 817–828, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J 21: 1743–1753, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannan RL, Kourembanas S, Flanders KC, Rogelj SJ, Roberts AB, Faller DV, Klagsbrun M. Endothelial cells synthesize basic fibroblast growth factor and transforming growth factor β. Growth Factors 1: 7–17, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Harrison CA, Gray PC, Vale WW, Robertson DM. Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab 16: 73–78, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Hirai R, Kaji K. Transforming growth factor β 1-specific binding proteins on human vascular endothelial cells. Exp Cell Res 201: 119–125, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Igarashi J, Shoji K, Hashimoto T, Moriue T, Yoneda K, Takamura T, Yamashita T, Kubota Y, Kosaka H. Transforming growth factor-β1 downregulates caveolin-1 expression and enhances sphingosine 1-phosphate signaling in cultured vascular endothelial cells. Am J Physiol Cell Physiol 297: C1263–C1274, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-β family members through Smad proteins. Eur J Biochem 267: 6954–6967, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol 19: 385–394, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Kim S, Lee Y, Seo JE, Cho KH, Chung JH. Caveolin-1 increases basal and TGF-β1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal 20: 1313–1319, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Kim S, Pak Y. Caveolin-2 regulation of the cell cycle in response to insulin in Hirc-B fibroblast cells. Biochem Biophys Res Commun 330: 88–96, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett 9: 195–220, 2004 [PubMed] [Google Scholar]
- 21. Kwon H, Jeong K, Hwang EM, Park JY, Hong SG, Choi WS, Pak Y. Caveolin-2 regulation of STAT3 transcriptional activation in response to insulin. Biochim Biophys Acta 1793: 1325–1333, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Kwon H, Jeong K, Pak Y. Identification of pY19-Caveolin-2 as a positive regulator of insulin-stimulated actin cytoskeleton-dependent mitogenesis. J Cell Mol Med, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lahtinen U, Honsho M, Parton RG, Simons K, Verkade P. Involvement of caveolin-2 in caveolar biogenesis in MDCK cells. FEBS Lett 538: 85–88, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Lee EK, Lee YS, Han IO, Park SH. Expression of Caveolin-1 reduces cellular responses to TGF-β1 through down-regulating the expression of TGF-β type II receptor gene in NIH3T3 fibroblast cells. Biochem Biophys Res Commun 359: 385–390, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lee H, Park DS, Wang XB, Scherer PE, Schwartz PE, Lisanti MP. Src-induced phosphorylation of caveolin-2 on tyrosine 19. Phospho-caveolin-2 (Tyr(P)19) is localized near focal adhesions, remains associated with lipid rafts/caveolae, but no longer forms a high molecular mass hetero-oligomer with caveolin-1. J Biol Chem 277: 34556–34567, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Massague J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J 19: 1745–1754, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, Rodriguez-Boulan E. Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem 274: 25708–25717, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-β signal transduction. J Cell Sci 114: 4359–4369, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Myoken Y, Kan M, Sato GH, McKeehan WL, Sato JD. Bifunctional effects of transforming growth factor-β (TGF-β) on endothelial cell growth correlate with phenotypes of TGF-β binding sites. Exp Cell Res 191: 299–304, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Neganova I, Lako M. G1 to S phase cell cycle transition in somatic and embryonic stem cells. J Anat 213: 30–44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O′Connor PM. Mammalian G1 and G2 phase checkpoints. Cancer Surv 29: 151–182, 1997 [PubMed] [Google Scholar]
- 32. Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-β 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA 97: 2626–2631, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ota T, Fujii M, Sugizaki T, Ishii M, Miyazawa K, Aburatani H, Miyazono K. Targets of transcriptional regulation by two distinct type I receptors for transforming growth factor-β in human umbilical vein endothelial cells. J Cell Physiol 193: 299–318, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Parat MO. The biology of caveolae: achievements and perspectives. Int Rev Cell Mol Biol 273: 117–162, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Parker S, Walker DS, Ly S, Baylis HA. Caveolin-2 is required for apical lipid trafficking and suppresses basolateral recycling defects in the intestine of Caenorhabditis elegans. Mol Biol Cell 20: 1763–1771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the golgi complex. J Biol Chem 274: 25718–25725, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Pece-Barbara N, Vera S, Kathirkamathamby K, Liebner S, Di Guglielmo GM, Dejana E, Wrana JL, Letarte M. Endoglin null endothelial cells proliferate faster and are more responsive to transforming growth factor β1 with higher affinity receptors and an activated Alk1 pathway. J Biol Chem 280: 27800–27808, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Jr, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol 22: 2329–2344, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem 276: 6727–6738, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Santibanez JF, Blanco FJ, Garrido-Martin EM, Sanz-Rodriguez F, del Pozo MA, Bernabeu C. Caveolin-1 interacts and cooperates with the transforming growth factor-β type I receptor ALK1 in endothelial caveolae. Cardiovasc Res 77: 791–799, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Schubert W, Sotgia F, Cohen AW, Capozza F, Bonuccelli G, Bruno C, Minetti C, Bonilla E, Dimauro S, Lisanti MP. Caveolin-1(-/-)- and caveolin-2(-/-)-deficient mice both display numerous skeletal muscle abnormalities, with tubular aggregate formation. Am J Pathol 170: 316–333, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9: 1149–1163, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Shmuel M, Nodel-Berner E, Hyman T, Rouvinski A, Altschuler Y. Caveolin 2 regulates endocytosis and trafficking of the M1 muscarinic receptor in MDCK epithelial cells. Mol Biol Cell 18: 1570–1585, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sowa G, Pypaert M, Fulton D, Sessa WC. The phosphorylation of caveolin-2 on serines 23 and 36 modulates caveolin-1-dependent caveolae formation. Proc Natl Acad Sci USA 100: 6511–6516, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sowa G, Xie L, Xu L, Sessa WC. Serine 23 and 36 phosphorylation of caveolin-2 is differentially regulated by targeting to lipid raft/caveolae and in mitotic endothelial cells. Biochemistry 47: 101–111, 2008 [DOI] [PubMed] [Google Scholar]
- 48. ten Dijke P, Hill CS. New insights into TGF-β-Smad signalling. Trends Biochem Sci 29: 265–273, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78: 67–74, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3: 11–20, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Wang XB, Lee H, Capozza F, Marmon S, Sotgia F, Brooks JW, Campos-Gonzalez R, Lisanti MP. Tyrosine phosphorylation of caveolin-2 at residue 27: differences in the spatial and temporal behavior of phospho-Cav-2 (pY19 and pY27). Biochemistry 43: 13694–13706, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, Choi AM. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 203: 2895–2906, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Wu X, Ma J, Han JD, Wang N, Chen YG. Distinct regulation of gene expression in human endothelial cells by TGF-β and its receptors. Microvasc Res 71: 12–19, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Xie L, Frank PG, Lisanti MP, Sowa G. Endothelial cells isolated from caveolin-2 knockout mice display higher proliferation rate and cell cycle progression relative to their wild-type counterparts. Am J Physiol Cell Physiol 298: C693–C701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem 280: 4864–4872, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Zaas DW, Swan ZD, Brown BJ, Li G, Randell SH, Degan S, Sunday ME, Wright JR, Abraham SN. Counteracting signaling activities in lipid rafts associated with the invasion of lung epithelial cells by Pseudomonas aeruginosa. J Biol Chem 284: 9955–9964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]