Abstract
An association between oxidative stress and muscle atrophy and weakness in vivo is supported by elevated oxidative damage and accelerated loss of muscle mass and force with aging in CuZn-superoxide dismutase-deficient (Sod1−/−) mice. The purpose was to determine the basis for low specific force (N/cm2) of gastrocnemius muscles in Sod1−/− mice and establish the extent to which structural and functional changes in muscles of Sod1−/− mice resemble those associated with normal aging. We tested the hypothesis that muscle weakness in Sod1−/− mice is due to functionally denervated fibers by comparing forces during nerve and direct muscle stimulation. No differences were observed for wild-type mice at any age in the forces generated in response to nerve and muscle stimulation. Nerve- and muscle-stimulated forces were also not different for 4-wk-old Sod1−/− mice, whereas, for 8- and 20-mo-old mice, forces during muscle stimulation were 16 and 30% greater, respectively, than those obtained using nerve stimulation. In addition to functional evidence of denervation with aging, fiber number was not different for Sod1−/− and wild-type mice at 4 wk, but 50% lower for Sod1−/− mice by 20 mo, and denervated motor end plates were prevalent in Sod1−/− mice at both 8 and 20 mo and in WT mice by 28 mo. The data suggest ongoing denervation in muscles of Sod1−/− mice that results in fiber loss and muscle atrophy. Moreover, the findings support using Sod1−/− mice to explore mechanistic links between oxidative stress and the progression of deficits in muscle structure and function.
Keywords: specific force, denervation, Sod1
reactive oxygen species (ROS) are by-products of normal cellular aerobic metabolism that can result in the oxidation of lipids, proteins, and nucleic acids. When ROS production exceeds antioxidant defenses, or when antioxidant mechanisms are impaired, the resulting state of oxidative stress can result in damage to cellular constituents, loss of cellular function, and cell death. ROS and oxidative stress have been implicated in the widely recognized wasting and weakness of skeletal muscle that develops with aging, commonly referred to as sarcopenia (15). Although sarcopenia undoubtedly results from complex interactions between both intrinsic and extrinsic factors, strong correlations have been reported between oxidative stress and the progression of muscle atrophy during aging (32, 34). Specifically, a mechanistic link between chronic oxidative stress in vivo and a loss of muscle mass and force is supported by studies of mice deficient in CuZn-superoxide dismutase (SOD) (Sod1−/−), a major antioxidant enzyme. Skeletal muscles from young Sod1−/− mice display elevated oxidative damage to proteins, lipids, and DNA compared with those of age-matched wild-type (WT) mice and muscle masses are significantly lower than those of WT mice as early as 6 mo of age (34). Muscle mass in the Sod1−/− mice is further reduced with age, and, by 20 mo, hindlimb muscle mass in Sod1−/− mice is nearly 50% lower than that in age-matched WT mice (34).
A major factor underlying the loss of muscle with aging in humans (7, 13) and rats (9, 21, 22, 26, 40) is the loss of entire motor units. In addition, for muscles of old rats, the maximum force of fast motor units is 70% that of comparable units in adult rats, whereas the force developed by slow motor units was nearly three times the adult value (21). These data suggest that motor unit remodeling with aging occurs by selective denervation of fast type 2 fibers with some reinnervation by collateral sprouting of nerves from fibers in slow motor units (4). The occurrence of a similar process in humans is supported by the grouping of slow type 1 fibers commonly reported in the muscles of the elderly (17, 19, 28). Aging results in changes at neuromuscular junctions (NMJ) (25, 42), including a decrease in muscles of old compared with adult animals in the number of nerve terminals (1), an overall decrease in the size of the motor end plates (33), fewer motor axon contacts per end plate (33), and decreases in the number, length, and density of postsynaptic folds (35). Thus the degeneration of nerve terminals at the motor end plate may constitute an ongoing process throughout life, with highly successful reinnervation in young animals by collateral sprouting (2, 4, 38), but impaired reinnervation in old animals (25, 33, 38). Similarly, degeneration of NMJ and the resultant denervation of muscle fibers have been proposed as the mechanism underlying the accelerated loss of muscle mass in Sod1−/− mice (18).
In addition to the skeletal muscle atrophy that develops with aging, force production is diminished in excess of what can be explained by the loss of muscle mass (3). Gastrocnemius (GTN) muscles of Sod1−/− mice also demonstrate decreased specific force normalized by muscle fiber cross-sectional area (CSA) (N/cm2) compared with muscles of WT mice (18). The mechanism underlying the decreased specific force by muscles of Sod1−/− mice is not known. The purpose of this study was to determine the basis for the weakness of GTN muscles of Sod1−/− mice and to establish the extent to which the structural and morphological changes that occur in muscles of Sod1−/− mice resemble those associated with the ongoing denervation process associated with normal aging. Based on the observations that muscles of Sod1−/− mice contain a high percentage of fragmented NMJs and only a tiny percentage of postsynaptic acetylcholine receptors showing normal colocalization with presynaptic motoneuron branches (18), we hypothesized that the weakness of whole muscles in Sod1−/− mice was due to the presence of a large population of functionally denervated fibers. The specific hypothesis was tested that the forces generated when muscles are activated by stimulation of the nerve or by direct stimulation of the muscle would not be different for WT mice, whereas muscles of Sod1−/− mice that demonstrate low specific forces would generate greater forces during direct muscle stimulation compared with nerve stimulation. We also performed histological analyses of GTN muscles of Sod1−/− and WT mice for evidence of denervated fibers in the Sod1−/− mice.
MATERIALS AND METHODS
Animals.
A total of 64 mice were analyzed between 1 and 22 mo of age for Sod1−/− mice and between 1 and 28 mo for WT mice. Sample sizes were between N = 6 and N = 14 for individual groups. The mice were obtained from the University of Texas Health Science Center at San Antonio (UTHSCSA) and have been previously described (18). In San Antonio, the mice were maintained under specific pathogen-free conditions in the heterozygous (Sod1+/−) state and backcrossed with C57BL/6J females (Jackson Laboratory, Bar Harbor, ME) for more than 20 generations. In the colony at UTHSCSA, the median lifespans of Sod1−/− and WT mice are ∼23 mo and ∼31 mo, respectively. For the present study, Sod1−/− mice were acquired at ∼6 or ∼18 mo of age and maintained under specific pathogen-free conditions in the Unit for Laboratory Animal Medicine at the University of Michigan (UM) until they were tested at 8–10 or 20–22 mo of age. Similarly, WT mice were acquired at ∼6, ∼18, or ∼26 mo and tested at 8–10, 20–22, or 28 mo, respectively. In addition, mice were generated in a UM facility from heterozygous breeders obtained from UTHSCSA, so that mice could be tested shortly after weaning without the risks of shipping. All procedures were approved by the University Committee on the Use and Care of Animals at UM and were in accordance with the Guide for Care and Use of Laboratory Animals (Public Health Service, 19965, National Institutes of Health Publication No. 85-23).
Contractile measurements.
In all cases, mice were anesthetized with initial intraperitoneal injections of avertin (tribromoethanol, 250 mg/kg) with supplemental injections given to maintain an adequate level of anesthesia during all procedures. Isometric contractile properties for GTN muscles were measured in situ. In anesthetized mice, the whole GTN muscle was isolated from surrounding muscle and connective tissue using great care not to damage the nerve and/or blood vessels during the dissection. A 4–0 silk suture was tied around the distal tendon, and the tendon was severed. The animal was then placed on a temperature-controlled platform warmed to maintain body temperature at 37°C. The hindlimb was securely tied to a fixed post with 4–0 monofilament nylon suture at the knee, and the foot was clamped to the platform. The distal tendon of the GTN muscle was then tied to the lever arm of a servomotor (model 305B, Aurora Scientific). A continual drip of saline warmed to 37°C was administered to the GTN muscle to maintain its temperature. The muscle was initially activated by stimulation of the tibial nerve using a bipolar platinum wire electrode. The voltage of single 0.2-ms stimulation pulses was adjusted to give a maximum isometric twitch. Subsequently, muscle length was adjusted to the optimal length (Lo) at which twitch force was maximal. With the muscle held at Lo, 300-ms trains of stimulus pulses were applied at increasing stimulation frequencies until the maximum isometric tetanic force (Po) was achieved. Subsequently, the same procedure was repeated, but rather than activating the muscle via the tibial nerve, a cuff electrode was placed around the proximal and distal ends of the muscle for stimulation.
After all force measurements, muscles were removed, and deeply anesthetized mice were killed by administration of a pneumothorax. GTN muscles were trimmed of their tendons, blotted, and weighed. Muscle fiber length was calculated by multiplying Lo by 0.45 (5). Total fiber CSA was calculated by dividing the muscle mass (mg) by the product of muscle fiber length (mm) and the density of mammalian skeletal muscle, 1.06 g/cm2. Specific Po (N/cm2) was calculated by dividing Po by total fiber CSA for each muscle. Immediately after muscle mass was measured, muscles were coated in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC), frozen in isopentane cooled by dry ice, and stored at −80°C until needed.
Muscle histological analysis.
Samples were sectioned at −20°C through the midbelly with a thickness of 12 μm, and fluorescent immunohistochemical staining was initiated the same day. For analysis of fiber number and fiber CSAs, fibers were identified by staining for myosin heavy chain and laminin to visualize fiber outlines. Frozen sections were rinsed with phosphate-buffered saline (PBS) to remove the freezing medium and then permeabilized in 0.2% Triton X-100 in PBS for 5 min. Sections were incubated overnight in blocking solution (Vector M.O.M. Mouse Ig Blocking Reagent) in a sealed chamber at 4°C and then soaked in M.O.M. dilutent (Vector) for 5 min. A second overnight incubation of the muscle sections was performed in the sealed chamber at 4°C with primary antibodies for type 1 myosin (mouse IgM; Alexis A4.840), type 2 myosin (mouse IgG; Thermo MS-1236-S), and laminin (rabbit IgG; Sigma L9393) in M.O.M. dilutent. Fluorescent labeling was completed by incubating sections in 1:1:1:300 solutions of secondary antibodies in M.O.M. dilutent (Vector) for 30 min in the dark at room temperature. Secondary Alexa Fluor antibodies, all from Invitrogen, were goat anti-mouse IgM (A-21426), goat anti-mouse IgG (A-21120), and goat anti-rabbit IgG (A-11008). Sections were mounted with Prolong Gold Mounting Medium without 4′,6-diamidino-2-phenylindole and imaged within 24 h on an Olympus BX-51 microscope. Adobe Photoshop CS4 was used to merge microscope images, and entire cross sections were then analyzed by an observer blinded to the identity of the sample for fiber number and CSAs using CyteSeer 2.0.4 (Vala Sciences, San Diego, CA).
Immunofluorescent staining with a neural cell adhesion molecule (NCAM)-specific antibody was performed to detect the presence of nerves and denervated skeletal muscle fibers. Although this antibody produces faint background staining in the connective tissue surrounding innervated muscle fibers, it specifically recognizes and brightly stains nerves and plasma membranes of denervated skeletal muscle fibers. Frozen sections were fixed with ice-cold methanol for 10 min and rinsed three times with PBS. Sections were blocked for 30 min at room temperature with PBS-0.05% Tween 20 (PBST) containing 20% calf serum (PBST-S) and then incubated overnight at 4°C with rabbit anti-NCAM (Chemicon International, Temecula, CA) primary antibody diluted in PBST-S. Following three washes in PBST, a 1-h room temperature incubation with Cy3-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratory) diluted in PBST-S was used for visualization of NCAM. After three washes in PBST, the sections were mounted with Prolong Gold Mounting Medium without 4′,6-diamidino-2-phenylindole and examined and photographed. After NCAM fluorescent images were acquired, coverslips were removed, and mounting medium was washed from the sections by incubation with five changes of PBST for 5 min each. Sections were blocked for 30 min with PBST-S at room temperature and incubated overnight at 4°C with rabbit anti-laminin (Chemicon International, Temecula, CA) primary antibody diluted in PBST-S. Following three washes in PBST, sections were incubated at room temperature for 1 h with Cy2-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratory) in PBST-S for visualization of laminin. Following a final three washes in PBST, the sections were mounted with Prolong Gold Mounting Medium, and the same areas of the sections that were previously examined for NCAM were photographed following laminin immunostaining.
NMJ immunohistochemistry.
Muscles were processed for acetylcholinesterase immunohistochemistry, as previously described (39). Muscles were sectioned longitudinally, with a thickness of 14 μm and mounted on SuperFrost glass slides. Sections were heated on a slide warmer for 10 min (55°C) and hydrated in distilled water (dH2O) for 5 min. After incubating in 20% sodium sulfate for 3 min, the sections were rinsed in dH2O, and, subsequently, a solution containing the esterase substrate 5-bromoindoxyl acetate, which stains motor end plates blue, was applied for 60 min at 37°C. The composition of this acetylcholinesterase solution was 4 mg 5-bromoindoxyl acetate, 0.3 ml ethanol, 63 mg potassium ferrocyanide, 50 mg potassium ferricyanide, and 30 ml 1× Trizma hydrochloride solution (Sigma Aldrich, St. Louis, MO). After rinsing in dH2O followed by PBS (0.1 M, pH 7.2) for 5 min, endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide for 10 min and rinsed in PBS. Nonspecific adherence of antisera was reduced by rinsing with PBS containing 0.1% Triton X-100, 1% normal goat serum, and 2% nonfat dry milk for 10 min. To detect nerve fibers, an antibody for neurofilament (Millipore, Billerica, MA), a major component of the axonal cytoskeleton, diluted in PBS containing 0.1% Triton X-100, was applied overnight in a humidified chamber at 22°C. Sections were incubated with goat anti-rabbit (Vectastain Kit, Vector Laboratories, Burlingame, CA) diluted in PBS containing 0.1% Triton X-100 for 1 h at 22°C. Following a PBS rinse, Vectastain ABC reagent (Vector Laboratories) containing avidin DH and biotinylated enzyme, was applied for 30 min. The chromogen was developed in 3,3′-diaminobenzidine solution (Vector Laboratories) for 30 s. The sections were rinsed in PBS, dehydrated with a series of ethanol and xylene rinses, and coverslipped with di-n-butyl-phthalate in xylene (DPX, Electron Microscopy Sciences, Hatfield, PA). Images were captured using a Spot-RT camera (Diagnostic Instruments, Sterling Heights, MI) attached to a Nikon Microphot-FXA microscope at ×20. Nerve to motor end-plate contact was determined by manually counting the number of motor end plates innervated by a nerve fiber (dark brown) and dividing by the total number of motor end plates (blue) present in each section.
Statistics.
Based on the large number of groups and the relatively tight distributions, rather than displaying all individual measurements, data are presented as means ± 1 SE to increase the clarity of the figures. Data for 1-, 8-, and 20-mo-old mice were compared by two-factor (genotype × age) ANOVA. When the ANOVA showed significant differences between groups, individual differences were established by Bonferroni post hoc analyses. One-way ANOVA was used to determine the effects of age for WT mice between 1 and 28 mo. Significance was set at P < 0.05.
RESULTS
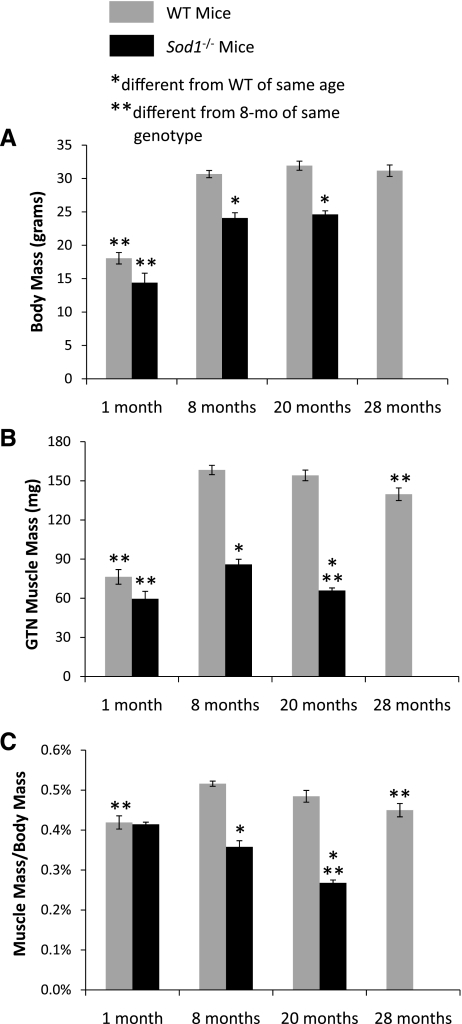
For young mice (1 mo), no differences between Sod1−/− and WT mice were observed for body mass (Fig. 1A) or GTN muscle mass, expressed either in absolute terms (Fig. 1B) or relative to body mass (Fig. 1C). For older mice, consistent with previous studies (18), body masses were ∼20% lower for Sod1−/− than WT mice (Fig. 1A). The lower body masses were observed for both 8- and 20-mo-old mice, with no effect of age for either group. Measurements of muscle mass showed substantial atrophy by 8 mo of age for GTN muscles of Sod1−/− mice that worsens by 20 mo (Fig. 1B). At 8 mo, GTN muscle masses were 50% lower for Sod1−/− compared with WT mice and decreased over 20% between 8 and 20 mo. The decrease in mass observed between 8 and 20 mo for GTN muscles of Sod1−/− mice was similar to the loss in mass for muscles of WT mice by 28 mo of age (Fig. 1B). When muscle mass was normalized by body mass, age-associated atrophy was observed for GTN muscles of both Sod1−/− and WT mice. Despite the significantly smaller body masses for the Sod1−/− mice, muscle mass normalized for body mass still showed a 25% decrease between 8 and 20 mo, which compared with a smaller, but statistically significant, 13% decrease for the WT mice between 8 and 28 mo (Fig. 1C).
Fig. 1.
Body mass (A) and gastrocnemius (GTN) muscle mass (B and C) are shown for wild-type (WT) and CuZnSOD-deficient (Sod1−/−) mice of varying ages. Body masses are expressed in g, and muscle masses are expressed both in mg (B) and as a percentage of body mass (C). Values are means ± 1 SE. Sample sizes are N = 8, 14, 11, and 8 for WT mice at 1, 8, 20, and 28 mo, respectively, and N = 6, 10, and 7 for Sod1−/− mice at 1, 8, and 20 mo, respectively. Significant difference from *WT value at the same age, **8-mo-old mice of the same genotype: P < 0.05.
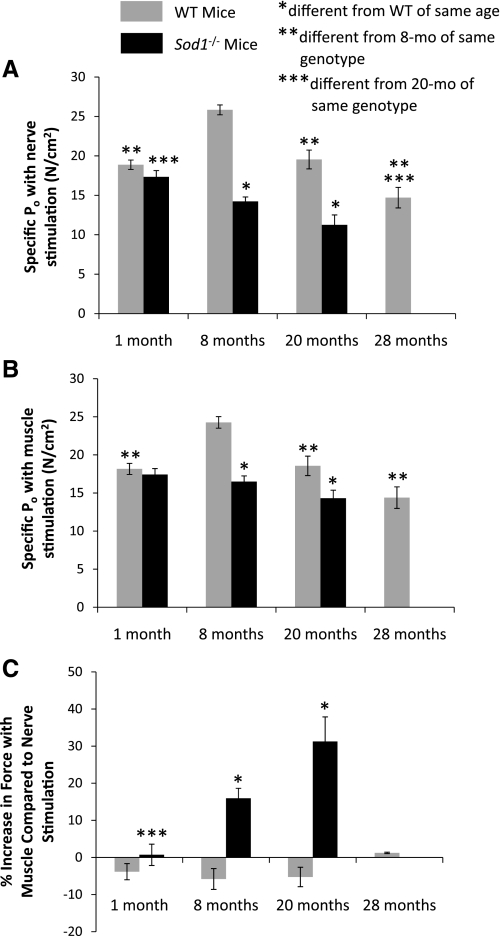
Consistent with the similarity in muscle sizes for young Sod1−/− and WT mice, force-generating capacity was also not different between the two genotypes, either when activated by stimulation through the nerve (Fig. 2A) or by directly stimulating the muscle (Fig. 2B). In contrast, by 8 mo of age, the muscles of Sod1−/− mice generated lower forces than those of WT mice. The lower forces were not simply a consequence of the smaller muscle masses, as evidenced by the observation that, when forces were normalized by muscle CSA, specific Po was lower for muscles of Sod1−/− mice than for those of WT mice. When stimulated through the nerve, specific Po for muscles of Sod1−/− mice was ∼45% lower than the value for WT mice at both 8 and 20 mo of age (Fig. 2A). For Sod1−/− and WT mice, GTN muscles demonstrated progressive muscle weakness with increasing age. Specific Po decreased by 35% between 1 and 20 mo for muscles of Sod1−/− mice and by 40% between 8 and 28 mo for WT mice (Fig. 2A), although specific Po may peak for Sod1−/− mice between 1 and 8 mo, in which case the magnitude of the decline in force with aging is underestimated by comparing the values at 1 mo and 20 mo of age. Consistent with our hypothesis, activating GTN muscles of WT mice by direct stimulation resulted in no change in force generation compared with the force elicited using nerve stimulation. Similarly, for 1-mo-old Sod1−/− mice, the response of the muscles to nerve and direct muscle stimulation was not different, whereas for 8- and 20-mo-old Sod1−/− mice, direct muscle stimulation increased force above values obtained using nerve stimulation by 16 and 31%, respectively (Fig. 2C).
Fig. 2.
Maximum isometric specific force (specific Po) values are shown for GTN muscles of WT and CuZnSOD-deficient (Sod1−/−) mice developed when the muscles were activated using nerve stimulation (A) or direct muscle stimulation (B). Specific Po is expressed in N/cm2. C: difference between the forces measured using these two methods of stimulation. Values are expressed as the percent increase in force developed with direct muscle stimulation compared with the force generated in response to nerve stimulation. Values are means ± 1 SE. Significant difference from *WT value at the same age, **8-mo-old mice of the same genotype, and ***20-mo-old mice of the same genotype: P < 0.05.
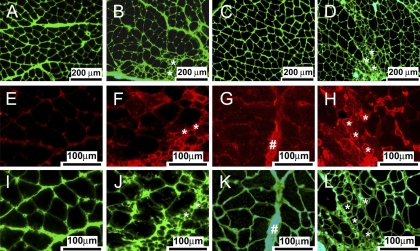
The lower GTN muscle masses in Sod1−/− compared with WT mice were explained largely by a decrease in the number of muscle fibers. Total fiber counts from whole muscle cross sections (Fig. 3A) indicated ∼5,000 fibers in GTN muscles of both Sod1−/− and WT mice at 1 mo of age (Fig. 3B). For WT mice, fiber number did not change between 1 and 20 mo, whereas dramatic fiber loss of nearly 45% was observed for Sod1−/− mice as GTN muscle mass decreased, such that fiber number was significantly lower in muscles of Sod1−/− compared with WT mice at both 8 and 20 of age (Fig. 3B). Accompanying the 13% decrease in GTN muscle mass by 28 mo for WT mice was an 11% decrease in fiber number compared with the pooled average for the younger mice, although the decrease did not reach statistical significance. Average fiber CSAs were not different between genotypes at any age. Despite the similarity in mean CSAs for fibers from muscles of Sod1−/− and WT mice, muscles of Sod1−/− mice showed increased numbers of small atrophic fibers. This increase in the number of atrophic fibers was especially evident in the 20-mo group, where histograms illustrating the distributions of muscle fiber CSAs showed that nearly 40% of the fibers in GTN muscles of Sod1−/− mice had CSAs <1,500 μm2 compared with only 20% of the fibers in WT mice within this size range (Fig. 3D).
Fig. 3.
A: representative section from a GTN muscle of an 8-mo-old WT mouse stained with antibodies for slow (red) and fast (blue) myosin and laminin (green) to designate the outline of fibers. Total number of fibers (B) and average cross-sectional areas (CSAs; μm2; C) in cross sections of GTN muscles of WT and CuZnSOD-deficient (Sod1−/−) mice of varying ages are shown. D: histograms show the distribution of CSAs for fibers in cross sections of GTN muscles of WT and CuZnSOD-deficient (Sod1−/−) mice at 20 mo of age. B and C: values are means ± 1 SE. Significant difference from *WT value at the same age, **8-mo-old mice of the same genotype, and ***20-mo-old mice of the same genotype: P < 0.05.
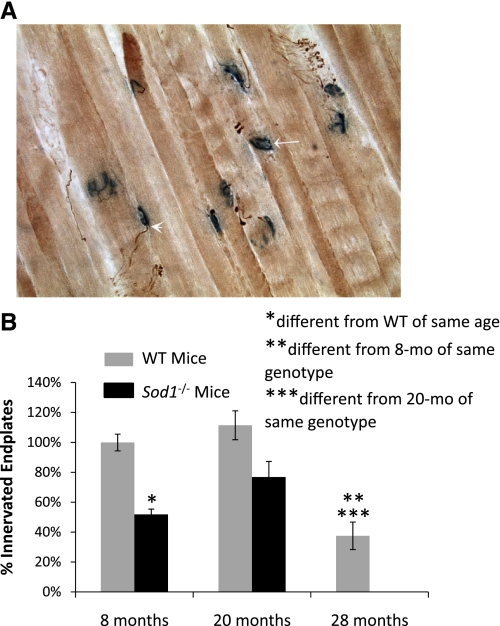
To investigate whether the small atrophic fibers observed in muscles of Sod1−/− mice were denervated, cross sections were stained for laminin and NCAM. Immunofluorescent images are shown in Fig. 4 and demonstrate that the atrophic fibers in the muscles of Sod1−/− mice, identified by the laminin boundaries, are largely positive for NCAM. Using NMJ immunohistochemistry, we quantified the number of motor end plates that showed colocalization of neurofilament with acetylcholinesterase staining as an indication of innervation of the end plate (Fig. 5). There was no difference in the number of innervated end plates for muscles of WT mice at 8 and 20 mo of age (Fig. 5B). In contrast, compared with the value obtained for muscles of 8-mo-old WT mice, there were 25–50% fewer innervated end plates in the muscles of Sod1−/− mice and even fewer in muscles of 28-mo-old WT mice (Fig. 5B).
Fig. 4.
Representative sections are shown for GTN muscles of 8-mo-old WT (A, E, and I) and CuZnSOD-deficient (Sod1−/−; B, F, and J) mice and 20-mo-old WT (C, G, and K) and Sod1−/− (D, H, and L) mice stained with antibodies for laminin (green) or neural cell adhesion molecule (NCAM; red). Scale bars represent 200 μm for A–D and 100 μm for E–L. Note the lack of NCAM-positive muscle fibers in sections from WT mice of either age. Nerve bundle was NCAM positive and is indicated by # in G. Note also the small atrophic fibers (indicated by * in B, F, J, D, H, and L) that are strongly NCAM positive and thus likely represent denervated fibers in sections of Sod1−/− mice at both 8 and 20 mo.
Fig. 5.
A: representative section is shown for GTN muscles assayed for acetylcholinesterase activity (blue) to visualize the motor end plate and costained with an antibody for neurofilament (brown) to visualize the motoneuron branches. Arrowhead indicates an end plate contacted by a nerve fiber, and a denervated end plate is indicated by the arrow. B: the number of motor end plates that are contacted by a nerve fiber was quantified and expressed as percentage of the total number of motor end plates in each section. Values are means ± 1 SE. Significant difference from *WT value at the same age, **8-mo-old mice of the same genotype, and ***20-mo-old mice of the same genotype: P < 0.05.
DISCUSSION
The major finding of the present study is that a significant number of fibers in GTN muscles of Sod1−/− mice do not contribute to force generation during nerve stimulation. The observation that direct stimulation of the muscles of Sod1−/− mice consistently increased force production over that generated using nerve stimulation suggests that a population of fibers is present in these muscles that are capable of producing force, but do not maintain a robust functional connection to the nerve. Our finding of a population of functionally denervated fibers in muscles of Sod1−/− mice is consistent with previous reports of morphological, as well as gene expression changes indicative of muscle-fiber denervation in CuZnSOD-deficient mice (18, 23). Our conclusion that muscle fibers in Sod1−/− mice undergo progressive denervation is supported by histological findings, including the presence of a population of small atrophic fibers positive for NCAM. NCAM, also known as the cluster of differentiation 56, is a glycoprotein normally expressed on the surface of neurons and skeletal muscle fibers. In innervated muscle fibers, NCAM expression is restricted to the NMJ, but, following denervation and during reinnervation, NCAM is abundantly expressed in nonsynaptic regions of the fibers (11). NCAM immunohistochemistry identified a population of myofibers in muscles of 8- and 20-mo-old Sod1−/− mice that would not be expected to respond to neural stimulation (8). Our observation of a lack of colocalization between neurofilament and acetylcholinesterase in substantial numbers of fibers in muscles of 8- and 20-mo-old Sod1−/− mice, as well as 28-mo-old WT mice, is also consistent with the presence of denervated fibers and with previous reports of large numbers of denervated motor end plates in CuZnSOD-deficient mice (18). Over three-fourths of NMJ sampled by Jang and colleagues (18) were categorized as denervated using fluorescently tagged α-bungarotoxin to visualize postsynaptic acetylcholine receptors. Although the present study identified fewer denervated end plates than Jang et al. previously reported, both studies showed dramatic effects of CuZnSOD deficiency on NMJ morphology.
During normal aging in human beings and rats, denervation of muscle fibers with limited reinnervation by collateral sprouting from neighboring motoneurons (21, 27) results in fiber loss and the accumulation of clusters of fibers of the same fiber type situated adjacent to one another (27, 29). Consistent with a similar process in the Sod1−/− mice, substantially fewer fibers were present in GTN muscles of Sod1−/− compared with WT mice in the present study by 8 mo of age, whereas fiber numbers were similar in Sod1−/− and WT mice early in life. In addition, Kostrominova (23) reported type 2a fiber grouping in muscles of Sod1−/− mice, suggesting that the denervation/reinnervation process in Sod1−/− mice may not be entirely distinct from the motor unit remodeling that occurs with normal aging in humans. While a loss of muscle fibers is the primary contributor to age-associated muscle atrophy (30), decreased CSA is also observed for the individual fibers that remain (14, 29). Similarly, an increase was observed in the present study in the number of small atrophic fibers in GTN muscles of Sod1−/− mice by 20 mo. Although denervation, fiber loss, and atrophy in Sod1−/− mice represent phenomena associated with normal aging, the state of partial innervation that exists for some muscle fibers in Sod1−/− mice, in which NMJ do not transmit action potentials but provide sufficient neural support to maintain the fiber's ability to generate force when directly activated, appears to be unique to the high oxidative stress environment of the CuZnSOD-deficient mice. In WT mice, muscles exhibit no difference in their response to nerve and muscle stimulation throughout the lifespan, despite the presence of denervated end plates in muscles of 28-mo-old WT mice.
Although the present findings indicate that the lower specific forces generated with nerve stimulation by GTN muscles of Sod1−/− compared with WT mice is due, in part, to the presence of a population of fibers that were not activated, using direct stimulation to activate these functionally denervated muscle fibers did not completely eliminate the deficit. The failure of direct stimulation to abolish the weakness of GTN muscles in Sod1−/− mice indicates that, in addition to the impact of the lack of CuZnSOD to disrupt NMJ, there are also detrimental effects within individual muscle fibers. Similarly, the weakness displayed by muscles of old WT mice with direct muscle stimulation suggests that contractile deficits may also exist within GTN fibers of old WT mice, as has been previously reported for individual flexor digitorum brevis, extensor digitorum longus, and soleus muscle fibers of mice (16, 20). The 30–40% deficits in isometric specific force observed in the present study during direct stimulation of whole GTN muscles of 8- and 20-mo-old Sod1−/− mice and 20- and 28-mo-old WT mice indicate that either individual fibers within these muscles also show 30–40% deficits in force generation, or most fibers generate normal forces while many fibers generate essentially no force. Some combination of fibers with a wide range of contractile deficits may also exist. To distinguish between these possibilities requires assessment of force generation of individual fibers.
Previous studies of single skinned fibers report effects of exposure to ROS that range from force reductions (6, 12), to force enhancement (12), to no effect (6, 24). This variability reflects, at least in part, a requirement of low levels of ROS in skeletal muscle for normal force generation (10, 36, 37). The association between high oxidative stress and profound weakness in skeletal muscle of Sod1−/− mice support the possibility of direct effects of oxidative stress and damage on force generation. Also consistent with a role of oxidative stress and damage in decreasing force generating capacity are the observations of deficits in specific force for GTN muscles of WT mice at 20 mo of age in the present study and published reports of elevated protein oxidation in muscles of mice of the same age and strain (34). Damage to myofibrillar proteins by increased levels of reactive oxygen and/or nitrogen species has previously been proposed to play a role in the age-related decline in skeletal muscle force generation (41). Oxidative modifications of contractile proteins may disrupt actin-myosin interactions, thereby reducing force-generating capacity of individual muscle fibers (31).
In summary, this study provides strong support for a process of denervation in the muscles of Sod1−/− mice, resulting in profound fiber loss and muscle atrophy between 1 and 8 mo of age. In addition, the deficits in specific force generation observed in the present study with direct stimulation for GTN muscles of 8- and 20-mo-old Sod1−/− mice and 20- and 28-mo-old WT mice suggest additional effects of elevated oxidative stress on individual muscle fiber function. Overall, the findings of the present study support previous reports of a direct role for oxidative stress as an in vivo mediator of the age-associated progression of deficits in muscle structure and function (18).
Perspectives and Significance
The progressive disruption of NMJs, loss of innervation, and ultimate fiber loss observed in Sod1−/− mice resemble, in some ways, the motor unit remodeling and loss known to occur with normal aging in rats and humans. The factors that initiate the loss of motor units with aging are not known, although Jang et al. (18) proposed that superoxide-induced NMJ degeneration represents a mechanism of sarcopenia. While the findings of the present study support an important role for oxidative stress to promote muscle fiber denervation, neither the present study nor previous studies (18, 23) of atrophy and weakness of muscles in Sod1−/− mice address whether cellular events within muscle fibers, in motoneurons, or both contribute in a causative manner to denervation process. The rapid development of muscle atrophy and weakness between 1 and 8 mo of age in Sod1−/− mice support the utility of this model to identify mechanistic links between oxidative stress and neuromuscular dysfunction.
GRANTS
This work was supported by National Institute on Aging Grant AG-020591.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.M.L., H.V.R., A.R., E.L.F., and S.V.B. conception and design of research; L.M.L., C.S.D., C.S.-R., and T.Y.K. performed experiments; L.M.L., C.S.-R., T.Y.K., H.V.R., A.R., E.L.F., and S.V.B. interpreted results of experiments; L.M.L., C.S.D., C.S.-R., T.Y.K., H.V.R., A.R., E.L.F., and S.V.B. edited and revised manuscript; L.M.L., C.S.D., C.S.-R., T.Y.K., H.V.R., A.R., E.L.F., and S.V.B. approved final version of manuscript; C.S.D., C.S.-R., T.Y.K., and S.V.B. analyzed data; C.S.D., C.S.-R., T.Y.K., and S.V.B. prepared figures; S.V.B. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to Elizabeth Armstrong, Mark Bolinger, Adriana Lingl, Douglas Montesano, and Emily Orban for help with data collection.
REFERENCES
- 1. Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve Suppl 5: S83–S87, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Barker D, Ip MC. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci 163: 538–554, 1966 [DOI] [PubMed] [Google Scholar]
- 3. Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown MC, Holland RL, Hopkins WG. Motor nerve sprouting. Annu Rev Neurosci 4: 17–42, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single- diaphragm fiber contractile apparatus. J Appl Physiol 90: 45–54, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36: 174–182, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cashman NR, Covault J, Wollman RL, Sanes JR. Neural cell adhesion molecule in normal, denervated, and myopathic human muscle. Ann Neurol 21: 481–489, 1987 [DOI] [PubMed] [Google Scholar]
- 9. Cederna PS, Asato H, Gu X, van der Meulen JH, Kuzon WM, Jr, Carlson BM, Faulkner JA. Motor unit properties of nerve-intact extensor digitorum longus muscle grafts in young and old rats. J Gerontol A Biol Sci Med Sci 56: B254–B258, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Coombes JS, Powers SK, Rowell B, Hamilton KL, Dodd SL, Shanely RA, Sen CK, Packer L. Effects of vitamin E and alpha-lipoic acid on skeletal muscle contractile properties. J Appl Physiol 90: 1424–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Covault J, Sanes JR. Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol 102: 716–730, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darnley GM, Duke AM, Steele DS, MacFarlane NG. Effects of reactive oxygen species on aspects of excitation-contraction coupling in chemically skinned rabbit diaphragm muscle fibres. Exp Physiol 86: 161–168, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol 18: 331–358, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Dupont-Versteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40: 473–481, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fulle S, Protasi F, Di TG, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol 39: 17–24, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol 178: 175–183, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand 115: 125–134, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 24: 1376–1390, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jennekens FG, Tomlinson BE, Walton JN. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci 14: 259–276, 1971 [DOI] [PubMed] [Google Scholar]
- 20. Jimenez-Moreno R, Wang ZM, Gerring RC, Delbono O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J 94: 3178–3188, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kadhiresan VA, Hassett CA, Faulkner JA. Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol 493: 543–552, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol 61: 737–746, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Kostrominova TY. Advanced age-related denervation and fiber-type grouping in skeletal muscle of SOD1 knockout mice. Free Radic Biol Med 49: 1582–1593, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol 546: 149–163, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci 50 Spec No: 91–95, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Larsson L, Ansved T. Effects of age on the motor unit. A study on single motor units in the rat. Ann N Y Acad Sci 515: 303–313, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol 50A: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci 72: 211–222, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol (Berl) 81: 377–381, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol 280: C540–C547, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Mansouri A, Muller FL, Liu Y, Ng R, Faulkner JA, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127: 298–306, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Messi ML, Delbono O. Target-derived trophic effect on skeletal muscle innervation in senescent mice. J Neurosci 23: 1351–1359, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med 40: 1993–2004, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Oki S, Desaki J, Ezaki T, Matsuda Y. Aged neuromuscular junctions in the extensor digitorum longus muscle of the rat as revealed by scanning electron microscopy. J Electron Microsc (Tokyo) 48: 297–300, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol 75: 1081–1087, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Reid MB, Moody MR. Dimethyl sulfoxide depresses skeletal muscle contractility. J Appl Physiol 76: 2186–2190, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Rosenheimer JL. Ultraterminal sprouting in innervated and partially denervated adult and aged rat muscle. Neuroscience 38: 763–770, 1990 [DOI] [PubMed] [Google Scholar]
- 39. Rubin A, Mobley B, Hogikyan N, Bell K, Sullivan K, Boulis N, Feldman E. Delivery of an adenoviral vector to the crushed recurrent laryngeal nerve. Laryngoscope 113: 985–989, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Sugiura M, Kanda K. Progress of age-related changes in properties of motor units in the gastrocnemius muscle of rats. J Neurophysiol 92: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Thompson LV. Age-related muscle dysfunction. Exp Gerontol 44: 106–111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson MH, Deschenes MR. The neuromuscular junction: anatomical features and adaptations to various forms of increased, or decreased neuromuscular activity. Int J Neurosci 115: 803–828, 2005 [DOI] [PubMed] [Google Scholar]