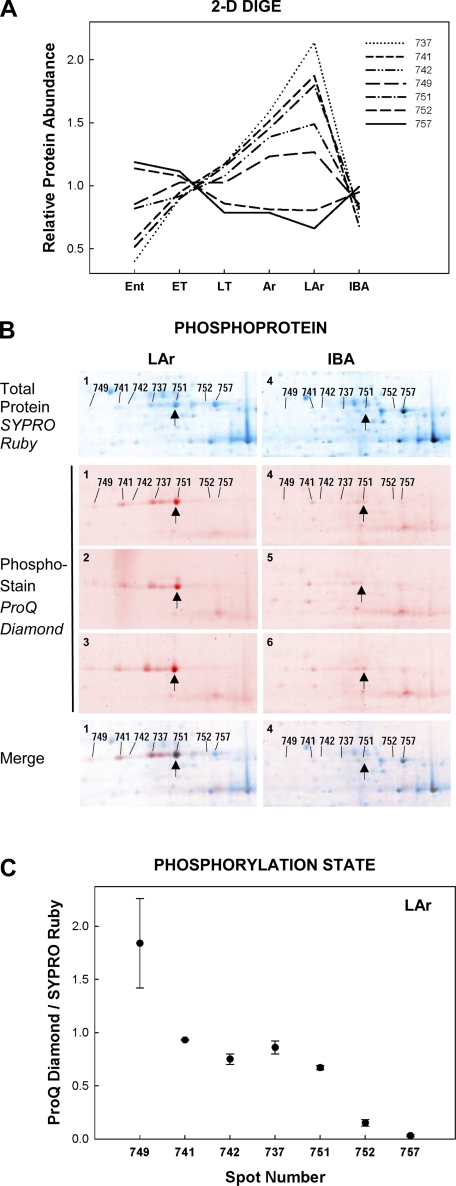
Fig. 7.
Patterns of relative protein abundance across winter hibernation states, defined in materials and methods, elucidates a mechanism of protein regulation during heterothermy. A: reciprocal Cy2-normalized abundance patterns of 7 protein spots all identified as PGM1 imply that this protein undergoes hibernation state-specific posttranslational modification. Phosphoprotein staining (ProQ Diamond) of 2D gels from n = 3 squirrels in each of 2 winter states [LAr vs. IBA (B)] supports a specific posttranslational modification, phosphorylation, of the more acidic spots (749, 741, 742, 737, 751) in the PGM1 train. B: false-color overlay to compare the SYPRO Ruby total protein stain (blue) with ProQ Diamond phosphoprotein stain (red) and merged images for LAr and IBA. A representative gel from each state poststained for total protein with SYPRO Ruby is labeled for each PGM1 spot. The PGM1 spot train region of each ProQ Diamond-stained gel (LAr 1–3, IBA 4–6) is also shown, along with 1 ProQ Diamond-SYPRO Ruby merged image from each state. Spot 751 is indicated (↑) in each image for comparison. The phospho-forms comprised a larger proportion of the overall PGM1 signature in LAr, while the dephosphorylated forms (752 and 757) were more prevalent in IBA. Quantification of phosphorylation state (ProQ Diamond/SYPRO Ruby staining intensity) is presented for each LAr gel (n = 3 gels stained in sequence, means ± SE; C). Staining intensity for both variables was normalized to the lowest-staining spot on each gel. The general increase in phosphorylation state moving left along the spot train (i.e., toward increasingly acidic protein spots) is expected if the posttranslational modification is indeed phosphorylation.