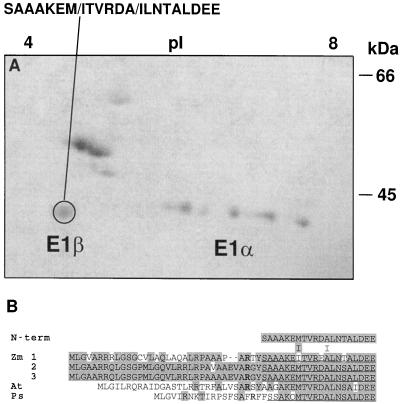
Figure 7.
N-terminal microsequencing of PDH subunits. A, Coomassie-blue-stained two-dimensional gel electrophoresis of highly purified maize mitochondrial PDC from etiolated shoots. The pI is indicated at the top and the size in kilodaltons is indicated to the right. The circled polypeptide was microsequenced from a replica-blot transferred to a PVDF membrane. At cycles 7 and 12 two residues were obtained (indicated by the slash). B, Comparison of the deduced amino acid sequence for the plant E1β subunits. Zm, Z. mays; At, Arabidopsis; Ps, P. sativum. Shading indicates amino acid identity. Gaps denoted by dashes were inserted to maximize homology. The N termini of the mature maize and pea polypeptides are underlined. Conserved Arg residues involved with peptide processing are indicated in bold type.