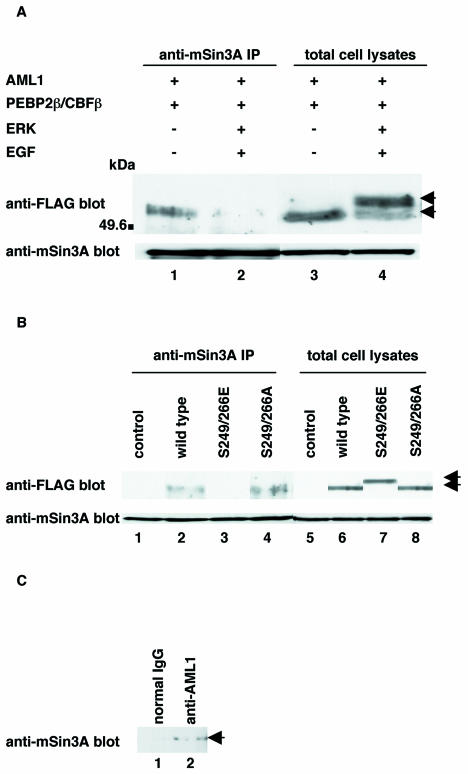
FIG. 1.
Analyses of the interaction between phosphorylated AML1 and mSin3A. (A) COS-7 cells were transfected with pME-FLAG-AML1 and pME-PEBP2β, together with pRc/CMV (lanes 1 and 3) or pCMVMK (lanes 2 and 4), starved in medium containing 0.1% FCS, treated for 5 min with 100 ng of EGF per ml plus 10% FCS (lanes 2 and 4) or left untreated (lanes 1 and 3), and harvested with lysis buffer. The cell lysates were precleared with protein G-Sepharose, mixed with anti-mSin3A antibody, and rotated for 2 h at 4°C. Then, mSin3A was recovered on protein G-Sepharose beads. The washed beads were subjected to SDS-PAGE, followed by Western blotting with anti-FLAG or anti-mSin3A antibody. Expression of each protein was monitored by using 60 μg of total cell lysates. +, present; −, absent; IP, immunoprecipitate; arrows, phosphorylated and unphosphorylated AML1. (B) COS-7 cells were transfected with pME18S, pME-FLAG-AML1, pME-FLAG-S249/266E, or S249/266A with pME-PEBP2β and harvested with lysis buffer. The cell lysates were precleared with protein G-Sepharose, mixed with anti-mSin3A antibody, and rotated for 2 h at 4°C. Then, mSin3A was recovered on protein G-Sepharose beads. The washed beads were subjected to SDS-PAGE, followed by Western blotting with the anti-FLAG or anti-mSin3A antibody. Expression of each protein was monitored by using 60 μg of total cell lysates. Arrows indicate wild-type and mutant AML1. (C) HEL cells were harvested with lysis buffer, and the cell lysates were precleared with protein G-Sepharose. The same amount of cell lysates was mixed with anti-AML1 antibody (Ab-1) or rabbit polyclonal IgG and rotated for 12 h; this was followed by recovery of AML1 on protein G-Sepharose beads. The washed beads were subjected to SDS-PAGE and Western blotting with anti-mSin3A antibody. Arrow, AML1.