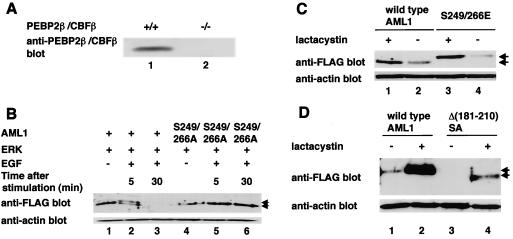
FIG. 8.
Phosphorylated AML1 and the AML1 mutants lacking interaction with mSin3A are degraded by proteasome in PEBP2β-deficient fibroblasts. (A) Embryonic fibroblasts from normal mice (lane 1) or PEBP2β-deficient mice (lane 2) were lysed, and 30 μg of cell lysates were subjected to SDS-PAGE and Western blotting with anti-PEBP2β antibody. (B) PEBP2β-deficient fibroblasts were cotransfected with (+) pME-FLAG-AML1 (lanes 1 to 3) or pME-FLAG-S249/266A (lanes 4 to 6), together with pCMVMK. The cells were starved in medium containing 0.1% FCS overnight and treated for 5 min with 100 ng of EGF per ml plus 10% FCS (lanes 2, 3, 5, and 6) or left untreated (lanes 1 and 4). They were harvested, and 30 μg of cell lysates was subjected to SDS-PAGE and Western blotting with anti-FLAG or anti-actin antibody. The EGF-treated cells were harvested after 5 (lanes 2 and 5) or 30 (lanes 3 and 6) min of EGF stimulation. Arrows, phosphorylated and unphosphorylated AML1. (C) PEBP2β-deficient fibroblasts were transfected with pME-FLAG-AML1 or pME-FLAG-S249/266E. The cells were treated with 10 μM lactacystin (lanes 1 and 3) or left untreated (lanes 2 and 4) overnight and harvested; 30 μg of cell lysates was subjected to SDS-PAGE and Western blotting with anti-FLAG or anti-actin antibody. (D) PEBP2β-deficient fibroblasts were transfected with pME-FLAG-AML1 or pME-FLAG-Δ(181-210)SA. The cells were treated with 10 μM lactacystin (lanes 2 and 4) or left untreated (lanes 1 and 3) overnight and harvested; 30 μg of cell lysates was subjected to SDS-PAGE and Western blotting with anti-FLAG or anti-actin antibody. Arrows in panels C and D indicate wild-type and mutant AML1.