Abstract
Idiopathic intracranial hypertension (IIH) is a condition of yet unknown aetiology affecting predominantly obese females of childbearing age. IIH is a diagnosis of exclusion as raised cerebrospinal fluid pressure may occur secondary to numerous other medical conditions. An atypical phenotype or an atypical disease course should alert the physician to reevaluate a presumed IIH-diagnosis. The authors report a case of a 32-year-old non-obese male with intracranial hypertension, secondary to a syphilitic central nervous system infection, initially misdiagnosed as being idiopathic. Upon relevant antibiotic treatment, signs and symptoms of elevated intracranial pressure resolved completely. Syphilis is a rare, but very important, differential diagnosis that in this case was clinically indistinguishable from IIH.
Background
This case very clearly demonstrates that idiopathic intracranial hypertension (IIH) is a diagnosis of exclusion and that it should not be accepted until even rare secondary causes have been explored. In our case, the intracranial hypertension was initially misdiagnosed as idiopathic due to low cerebrospinal fluid (CSF) white blood cell (WBC) count. Although serological markers of syphilis indicated severe infection load the clinical signs were sparse and initially misinterpreted as Scarlet fever. During the recent years, syphilis, although not yet recognised in many clinical settings, has had a strong resurgence in developed countries. As syphilis is a great mimicker the case should alert clinicians that the diagnosis should be considered in a broader spectrum of clinical presentations.
Case presentation
A 32-year-old male presented with blurred vision in his right eye and persistent bi-frontal, retro-bulbar headache during 2½ week. The headache was constant and extending in intensity from very mild to severe attacks of throbbing pain of 5–10 min duration, occurring 3–5 times a day. He complained of photophobia, photopsia and bilateral transient visual obscurations.
Medical history included weekly episodes of mild, holocranial tension-type headache and periodic neck pain after a horseback riding accident 7 years earlier. He was otherwise healthy.
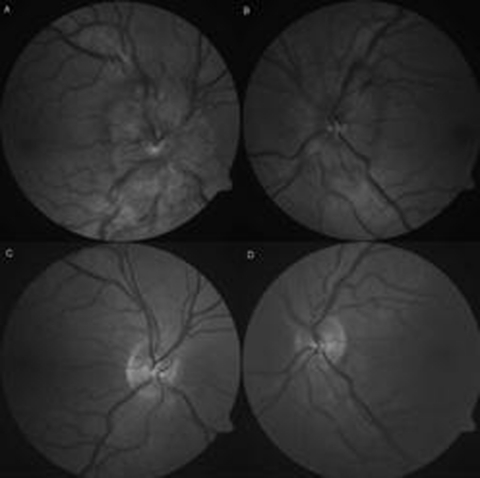
On examination, visual acuity was 10/20 on the right eye and 26/20 on the left. Ishihara colour vision was normal and no relative afferent pupillary defect (RAPD) was found. Visual fields (Humphrey 30-2) showed enlarged blind spots bilaterally (figure 1) and eye motility examination disclosed subtle right sixth nerve palsy. Slit lamp examination was normal showing no inflammatory cells in the anterior chambers or vitreous. Funduscopy revealed bilateral optic disc swelling, peripapillary haemorrhages and chorioretinal folds in the right posterior pole (figure 2).
Figure 1.
Visual fields (Humphrey 30-2) at time of diagnosis. Bilaterally enlarged blind spots.
Figure 2.
(A, B) At time of diagnosis. Pronounced bilateral papilloedema with blurred disc margins extending into the upper and lower temporal vascular arcades, peripapillary haemorrhages and moderate retinal vein congestion. (C, D) Complete resorption 7 months later.
General and neurological examination was normal. Cerebral MRI with gadolinium was unremarkable, optic nerves were normal with no signs of oedema or enhancement. Venous sequences excluded venous sinus thrombosis.
Standardised lumbar pressure measurement (relaxed decubital position with legs extended) revealed a raised CSF pressure of 28.5 cmH2O (normal range <25 cmH2O).1 CSF analysis demonstrated WBC 5×106/l (≤3×106), protein 0.44 g/l (0.15–0.50 g/l) and glucose 3.3 mM (2.2–3.9 mM). Blood samples were normal without signs of infection (WBC 5.9×106/l, C-reactive protein 6 mg/l). The patient was non-obese, weight 93 kg, height 1.93 m (body mass index 25 kg/m2).
Initially the diagnosis of IIH was assumed and treatment with acetazolamide 1500 mg daily was initiated.
The atypical phenotype, though, led to a second and more detailed interview revealing a history of homosexuality, 6 months of night sweats and an episode of arthralgia, nuchal rigidity, generalised skin rash and tonsillitis 2 months earlier (diagnosed as Scarlet fever by his general practitioner and treated with penicillin V 2.4 million units a day for 10 days).
Thorough investigation for secondary causes revealed strongly positive syphilis serology indicating acute and active infection: Wasserman (WR) 8, rapid plasma reagin (RPR) 64, Treponema pallidum flagel IgG (AF-G) 10, T pallidum flagel IgM (AF-M) 4 and fluorescens treponema antibodies (FTA-ABS) 3.03. HIV test was negative and additional blood tests were normal.
A second spinal tap to confirm the clinical diagnosis of neurosyphilis was inconclusive. At this point there was no pleocytosis. WR, AF-G, AF-M, FTA-ABS were negative. CSF protein was, however, elevated (0.63 g/l) and the patient thus fulfilled the diagnostic criteria of neurosyphilis (table 1).
Table 1.
Diagnostic criteria for neurosyphilis16
| CNS or ophthalmic signs or symptoms, plus serologic evidence (positive non-treponemal and treponemal test results) for syphilis infection plus one of the following: |
| Positive VDRL*-CSF |
| Increased CSF protein (> 40 mg/dl) |
| Increased CSF WBC count (>5 mononuclear cells/µl) |
VDRL is a non-treponemal test similar to RPR.
CSF, cerebrospinal fluid; CNS, central nervous system; VDRL, Venereal Disease Research Laboratory; WBC, white blood cell.
Differential diagnosis
-
▶
Inflammatory optic neuritis with papillitis
-
▶
Optic perineuritis
-
▶
Uveitis.
Treatment
Treatment was intravenous penicillin G 18 million units a day for 14 days.
Outcome and follow-up
Decreasing and eventually normalised serum values at subsequent follow-up visits indicated a sufficient therapeutic response (WR, RPR and AF-M=0 after 4 months).
Clinical improvement was significant almost immediately after initiation of antibiotic treatment. After 3 months, only habitual mild tension-type headache remained. Visual disturbances were completely resolved. Visual acuity was normalised (>20/20 on the right and 20/26 on the left eye), and eye motility was full. The papilloedema had resolved (apart form a small peripapillary neuroretinal oedema in the right eye) and visual fields showed only slightly enlarged blind spots bilaterally. Within a year, fundi and visual fields normalised completely.
Discussion
Syphilitic infection is classically divided into four stages. The primary stage is marked by genital or oral chancres. Skin rash and mucous membrane lesions characterise the secondary stage that might include fever, sore throat, fatigue, anorexia and weight loss. The latent stage of syphilis is asymptomatic and can last for years. If left untreated 15% eventually enter the tertiary stage characterised by infiltrative tumours (gumma) that mainly affect the cardiovascular system (80–85%) and the central nervous system (CNS) (5–10%).
Although neurosyphilis is often referred to as tertiary syphilis, CNS involvement can occur at any time in the course of infection.2 Neuroinvasion (dissemination of Treponema pallidum to the CNS) is believed to occur even before manifestation of the primary stage and may cause symptomatic disease within the first weeks or months of infection. Early forms of neurosyphilis primarily involve the meninges, CSF and CNS vasculature in contrary to parenchyma involvement of late neurosyphilis.3 4 Studies suggest that early but insufficient antibiotic treatment, as in the presented case, increases the risk of early neurosyphilis.2 5
In the present case, the serologic tests and patient history indicate a secondary stage of syphilis. Symptoms of headache, neck stiffness and photophobia in combination with neuro-ophthalmological signs of raised intracranial pressure (ICP) and documented high ICP strongly suggest early involvement of the CNS–probably a meningeal infection–causing a secondary intracranial hypertension. This hypothesis is supported by the prompt and sustained remission upon sufficient antibiotic treatment.
Although CNS analyses failed to detect syphilis antibodies the patient, due to an elevated protein concentration, met the diagnostic criteria of neurosyphilis.
The presenting low visual acuity in the right eye was not associated with signs of unilateral optic neuropathy (colour vision defects, RAPD or visual field defects other than an enlarged blind spot). The visual loss was thus regarded as purely retinal caused by subretinal macular oedema secondary to the papilloedema. The presenting chorioretinal folds and subsequent neuroretinal oedema supported this interpretation.6 Unfortunately, a confirming optical coherence tomography or fluorescence angiography was not performed. Visual function of the left eye was intact apart from an enlarged blind spot. Bilateral optic disc swelling in the absence of signs of optic neuropathy supports the diagnosis of papilloedema due to raised ICP and rules out the differential diagnosis of inflammatory optic neuritis with papillitis where signs of optic neuropathy would have been expected.
Optic perineuritis (nerve sheaths involved without inflammation of the nerve itself) might spare the visual acuity and visual fields apart from an enlarged blind spot.7 Bilateral optic perineuritis with optic disc swelling has been reported with signs indistinguishable from those of papilloedema.8 In the present case, however, contrast enhanced MRI showed no signs of optic nerve or nerve sheath inflammation, and optic perineuritis would fail to explain the high ICP and the related symptoms.
A third differential diagnosis is bilateral uveitis with papilloedema. Slit lamp examination however revealed no inflammatory cells in the anterior chamber or vitreous as would have been expected in this case with severe papilloedema.
Intracranial hypertension in relation to syphilitic has only been described in two case reports from 1987 and 2009. Unlike our case both of these patients displayed marked CSF pleocytosis.9 10 In addition to these cases, a retrospective study of 18 patients fulfilling the criteria of neurosyphilis, high ICP (50 cmH20) was demonstrated in one patient. Headache, impaired vision, swollen discs and tinnitus were noted in several others (opening pressures were not measured).11
The present case of secondary intracranial hypertension, initially misdiagnosed as idiopathic, clearly demonstrates the importance of eliminating any secondary aetiology before a diagnosis of IIH is applied. The physician should in particular be alert if the syndrome appears in an unusual phenotype, in this case male sex and normal body weight.
It is of great importance that clinicians realise that syphilis has had a strong reappearance during the recent years. Especially among homosexual males the incidence has exploded. In Denmark (population 5.5 million), the annual incidence of syphilis increased fivefold from 77 to 383 over a 4 year period from 2006 to 2010. The majority of cases (66.3%) were found among men who have sex with men (Department of Epidemiology, Statens serum institut: www.ssi.dk). This rapid increase is in line with reports from other Western countries.12–15
As syphilis is well-known to present with a wide variety of neurological and ophthalmological symptoms it is important to be aware of this diagnosis in any unusual disease presentation.
Learning points.
-
▶
Secondary causes of IIH may be occult showing no or only sparse serological and clinical signs.
-
▶
An atypical phenotype or an atypical disease course should alert the physician to reevaluate a presumed IIH-diagnosis.
-
▶
Syphilis is a great mimicker in clinical medicine. It has had a strong resurgence during the recent years and the diagnosis should be considered in a broad-spectrum of clinical presentations.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59:1492–5 [DOI] [PubMed] [Google Scholar]
- 2.Hook EW, 3rd, Marra CM. Acquired syphilis in adults. N Engl J Med 1992;326:1060–9 [DOI] [PubMed] [Google Scholar]
- 3.Marra CM. Update on neurosyphilis. Curr Infect Dis Rep 2009;11:127–34 [DOI] [PubMed] [Google Scholar]
- 4.Chahine LM, Khoriaty RN, Tomford WJ, et al. The changing face of neurosyphilis. Int J Stroke 2011;6:136–43 [DOI] [PubMed] [Google Scholar]
- 5.Ghanem KG. REVIEW: Neurosyphilis: A historical perspective and review. CNS Neurosci Ther 2010;16:e157–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoye VJ, 3rd, Berrocal AM, Hedges TR, 3rd, et al. Optical coherence tomography demonstrates subretinal macular edema from papilledema. Arch Ophthalmol 2001;119:1287–90 [DOI] [PubMed] [Google Scholar]
- 7.Purvin V, Kawasaki A, Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol 2001;119:1299–306 [DOI] [PubMed] [Google Scholar]
- 8.Low GS, Edis RH. Syphilitic perioptic neuritis mimicking papilloedema. Med J Aust 2009;191:236. [DOI] [PubMed] [Google Scholar]
- 9.Bakchine S, Mas JL, Bousser MG. Syphilitic meningitis masquerading as pseudotumor cerebri. Arch Neurol 1987;44:473. [DOI] [PubMed] [Google Scholar]
- 10.Cooper S, Razvi S, Alani A, et al. Syphilis presenting with headache and papilloedema. BMJ Case Rep 2009;2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CC, Leslie DE, Spelman D, et al. Symptomatic and asymptomatic early neurosyphilis in HIV-infected men who have sex with men: a retrospective case series from 2000 to 2007. Sex Health 2011;8:207–13 [DOI] [PubMed] [Google Scholar]
- 12.HIV and Sexually Transmitted Infections Department Health Protection Agency Table 2: Total number of STI diagnoses and other episodes of care seen at genitourinary medicine clinics in England: 2001–2010. London: Health Protection Agency, 2011:1–9 http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1215589013908 (accessed 27 October 2011). [Google Scholar]
- 13.Center for Disease Control and Prevention (CDC) Sexually Transmitted Disease Surveillance, 2009. Atlanta, Georgia, USA: Department of Health and Human Services, 2010 [Google Scholar]
- 14.Jakopanec I, Grjibovski AM, Nilsen Ø, et al. Syphilis epidemiology in Norway, 1992-2008: resurgence among men who have sex with men. BMC Infect Dis 2010;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velicko I, Arneborn M, Blaxhult A. Syphilis epidemiology in Sweden: re-emergence since 2000 primarily due to spread among men who have sex with men. Euro Surveill 2008;13 [DOI] [PubMed] [Google Scholar]
- 16.Sexually transmitted diseases treatment guidelines 2002 Centers for disease control and prevention. MMWR Recomm Rep 2002;51:1–78 [PubMed] [Google Scholar]