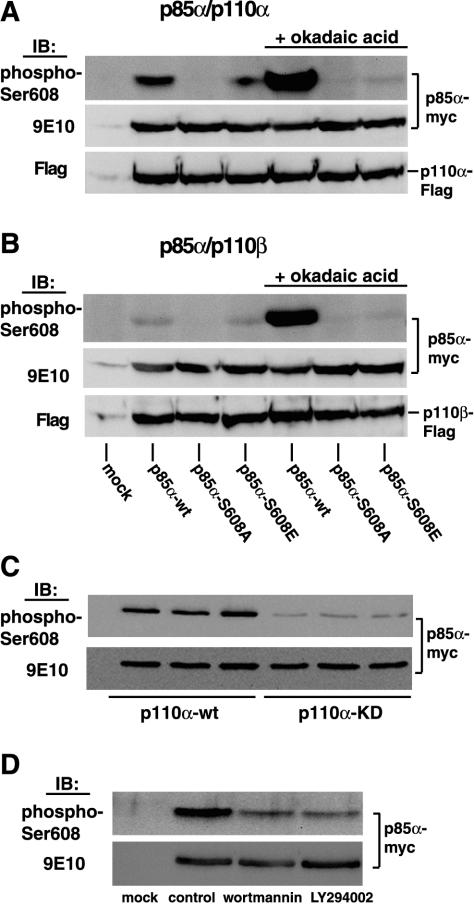
FIG. 2.
Phosphorylation of Ser608 after coexpression with either p110α or p110β in HEK 293 cells. (A) Myc-tagged p85α (wild type or Ser608 mutants) was coexpressed with Flag-tagged p110α in HEK 293 cells. Cells were lysed, and lysates were immunoprecipitated with antibody 9E10. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with the anti-phospho-Ser608 antibody. In some experiments, cells were treated with 500 nM okadaic acid for 45 min at 37°C prior to lysis. Uniform expression of Myc-tagged p85α and Flag-tagged p110 was confirmed after stripping of the blot and reprobing with antibody 9E10 and the Flag-M2 antibody, respectively (lower panels). (B) The same experiments as those described for panel A, except that p85α was cotransfected with p110β. (C) Cells were transfected with Myc-tagged p85α and either wild-type Flag-tagged p110α (p110α-wt) or catalytically inactive Flag-tagged p110α (p110α-KD [kinase dead]) and were further processed as described above. IB, immunoblot. (D) Cells were cotransfected with Myc-tagged p85α and Flag-tagged p110α. Transfected cells were treated with either 100 nM wortmannin or 30 μM LY294002 for 45 min at 37°C and were further processed as described above.