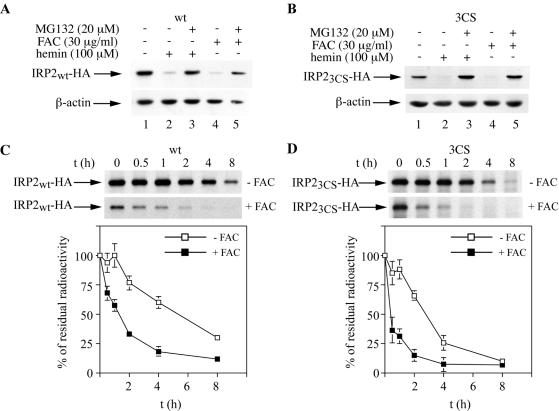
FIG. 2.
Iron-dependent turnover of wild-type IRP2 (IRP2wt) and IRP23CS. (A and B) Cells expressing IRP2wt (A) or IRP23CS (B) were treated overnight with 100 μM hemin (lanes 2 and 3) or 30 μg of FAC/ml (lanes 4 and 5) in the absence or presence of 20 μM MG132, and lysates were subjected to Western blotting with an anti-HA (top) or anti-β-actin (bottom) antibody. (C and D) Pulse-chase of IRP2wt (C) and IRP23CS (D) in the absence (top) or presence (bottom) of FAC. Control (untreated) or iron-loaded (pretreated overnight with 30 μg of FAC/ml) cells were metabolically labeled for 2 h with [35S]methionine-cysteine. Subsequently, the control or iron-loaded cells were chased for the indicated time intervals in cold medium either in the absence or in the presence of 30 μg of FAC/ml, respectively. Cytoplasmic lysates (500 μg) were subjected to quantitative immunoprecipitation with 1 μg of a purified polyclonal anti-HA antibody (Santa Cruz). Immunoprecipitated proteins were analyzed by SDS-PAGE on a 7.5% gel and visualized by autoradiography (arrows). The radioactive bands were quantified by phosphorimaging. In the graphs below the gels, the percentage of residual radioactivity from three independent experiments (mean ± standard deviation) is plotted against time.