Abstract
BACKGROUND:
Malaria and intestinal helminths are parasitic diseases causing high morbidity and mortality in most tropical parts of the world, where climatic conditions and sanitation practices favor their prevalence. The aim of this study was to determine the prevalence and possible impact of falciparum malaria and intestinal helminths co-infection among school children in Kajola, Osun state, Nigeria.
METHODS:
Fresh stool and blood samples were collected from 117 primary school children age range 4-15 years. The stool samples were processed using both Kato-Katz and formol-ether concentration techniques and microscopically examined for intestinal parasitic infections. Blood was collected by finger prick to determine malaria parasitemia using thick film method; and packed cell volume (PCV) was determined by hematocrit. Univariate analysis and chi-square statistical tests were used to analyze the data.
RESULTS:
The prevalence of Plasmodium falciparum, intestinal helminth infections, and co-infection of malaria and helminth in the study were 25.6%, 40.2% and 4.3%, respectively. Five species of intestinal helminths were recovered from the stool samples and these were Ascaris lumbricoides (34.2%), hookworm (5.1%), Trichuris trichiura (2.6%), Diphyllobothrium latum (0.9%) and Trichostrongylus species (0.9%). For the co-infection of both malaria and intestinal helminths, females (5.9%) were more infected than males (2.0%) but the difference was not statistically significant (p = 0.3978). Children who were infected with helminths were equally likely to be infected with malaria as children without intestinal helminths [Risk Ratio (RR) = 0.7295]. Children with A. lumbricoides (RR = 1.359) were also likely to be infected with P. falciparum as compared with uninfected children.
CONCLUSIONS:
Asymptomatic falciparum malaria and intestinal helminth infections do co-exist without clinical symp-toms in school children in Nigeria.
KEYWORDS: Malaria, Helminth, Co-infection, Nigeria
Malaria remains one of the most intractable public-health problems and is widely co-endemic with intestinal helminth infections, especially in children who live in the endemic regions of Sub-Saharan Africa resulting in a high rate of co-infection. In the last two decades, malaria epidemics have increased in frequency and intensity in most Sub-Saharan African populations due to the emergence of drug resistance Plasmodium.1 Whereas the prevalence of symptomatic infection is well documented, there is a paucity of data on asymptomatic infection. Many asymptomatic infections go undetected and untreated while causing little or no clinical manifestation. The extent of asymptomatic parasitemia is inversely related to a population's susceptibility to clinical disease,2–3 but more importantly, asymptomatic people are major reservoirs of infection.4,5
Malaria and helminths infections are widespread and they both have similar geographical distribution in developing countries with anaemia being a possible consequence. It is estimated that over a third of the world's population, mainly those individuals living in the tropics and subtropics, are infected by parasitic intestinal helminths or one or more of the species of Plasmodium.6 Plasmodium falciparum is responsible for a high burden of disease and a loss of growth in endemic countries estimated to be as high as 1.3% of gross domestic product per year.1 The threat of malaria continues through at least the first 5 years of life before most children living in endemic regions develop immunity sufficient to suppress severe pathogenesis.7–8 The major soil transmitted helminths (Ascaris lumbricoides, hookworm and Trichuris trichiura), coupled with schistosomiasis are responsible for more than 40% of the worldwide morbidity from all tropical infections, excluding malaria.9 Other intestinal helminths of medical importance to man include Trichinella spiralis (trichinosis), Enterobius vermicularis (pinworm) and Strongyloides stercoralis (Cochin-china diarrhea).
Concomitant parasitic infections could induce modifications of the specific immune response to each pathogen and thus modification of clinical expression.10 Helminths can therefore either ameliorate11 or exacerbate10,12 malaria severity. Young children from rural areas are principal victims of malaria,13 intestinal helminths,14 and anemia.15 In Nigeria, malaria and helminths infections are reportedly endemic and pose a significant health problem among children.16–18 In Lagos Nigeria, a prevalence of 48.6% was recorded for intestinal helminths and falciparum malaria among children living in the urban area.19 Also, prevalence ranging from 17.6% to 83% has been reported for intestinal helminths in rural areas of Nigeria.20–21 In Osogbo, the prevalence of clinical malaria among children has previously been reported to be about 30%,22 while intestinal helminths infection among school age children has not been well documented.
An interaction between helminths and malaria could work in either direction. Helminths infection may alter susceptibility to clinical malaria or malaria may influence the clinical consequences of helminth infection. Children infected either with intestinal helminths or S. haematobium are said to be more susceptible to acute malaria attacks.23–24 An increased likelihood of falciparum malaria was also observed in patients with helminths in a low-transmission area.25 Better quantification of the geographic extent of co-infection is an area that needs and deserves more critical investigation. The present study being the first study in this geographical location was designed to determine the prevalence and impact of malaria and helminth co-infection in children living in a setting where malaria is endemic. The results of this study could provide valuable information to local health authorities for improving existing control strategies
Methods
Study area and population
Kajola is a rural area in Atakunmosa west local government area of Osun state. It is about 10 km away from Osogbo, the state capital of Osun State Nigeria. It has a population of about 10,000 inhabitants. There is one public primary school, one secondary school and one primary health center in the community. The majority of the inhabitants are farmers. The study was carried out from May to August 2009, which spanned the rainy season.
The study subjects were primary school pupils with age range of 3 to 16 years. All children that were willing to be part of the study were selected. The inclusion criteria for the study included: (1) children aged 8-16 years; (2) parents or guardians gave written informed consent; and (3) children lived in the study area. Before samples were collected, demographic data such as sex, age, weight, and name of subject were recorded. Subjects diagnosed with malaria or intestinal helminths were treated appropriately. Ethical clearance for the work was obtained from the Osun State ministry of Health and Ministry of Education Ethical Committee (INS/EST/90).
Detection and quantification of malaria parasites
Blood samples were collected by finger prick for the determination of P. falciparum para-sitemia. Thick blood films were prepared, airdried, giemsa-stained, and observed under the microscope for identification and quantification of malaria parasites. Malaria parasites were counted against 200 leukocytes, and counts were expressed as the number of parasites per microliter of blood, assuming an average leukocyte count of 8,000 cells/μl of blood.26
Determination of packed cell volume
For packed cell volume (PCV, %), microhematocrit tubes filled with blood were centrifuged in a microhematocrit rotor for 5 min at 10,000 g. PCV values ≤31% were considered as anemia, which was further classified as mild (21–30%), moderate (15-20%), or severe (≤15%).13
Detection and quantification of helminths
Clean plastic containers were distributed for stool collection, and instructions were given for proper collection. The formol-ether concentration technique was employed and Kato-Katz thick smear technique was used for quantitative determination of helminths ova.27 Stool samples were processed within 12 hours of collection and examined microscopically within 1 hour of preparation to avoid missing hookworm ova. The intensity of infection was expressed as the number of eggs per gram of feces (epg). Water- or urine-contaminated stools were rejected.
Statistical analysis
Statistical analysis was done using JMP version 5 for windows North Carolina, USA. For uni-variate analysis, frequencies were compared using the Fisher's exact tests. Prevalence of malaria, helminth and co-infection were compared using χ2tests. Two sided p values < 0.05 indicated statistical significance.
Results
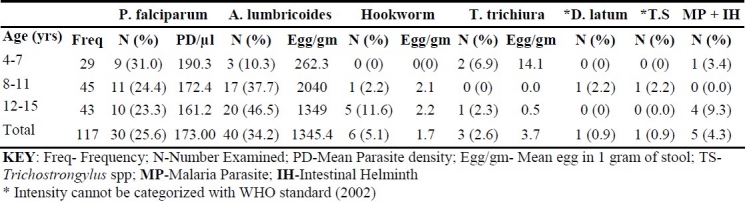
One hundred and seventeen stool and blood specimens collected from primary school children aged 4 up to 15 years were microscopically investigated in order to determine the prevalence of intestinal helminths and malaria infection among the children. The mean age of the study population was 9.9 ± 3.1 years while the mean height was 127 ± 14.0 cm and the mean weight was 25.5 ± 6.7 kg. The mean PCV of the study population was 33.6 ± 3.4 g/dL and none of the subjects had severe or moderate anaemia while 15 (12.8%) children had mild anemia. No association was observed between mild anemia and falciparum malaria or intestinal helminth infection. Table 1 shows the prevalence of intestinal helminth and falciparum malaria parasite in the study subjects according to age group. The prevalence of malaria parasite in the study was 25.6% while that of intestinal helminth was 40.2%. Five species of helminths were recovered from the stool samples and these were Ascaris lumbricoides (34.2%), hookworm (5.1%), Trichuris trichiura (2.6%), Diphyllobothrium latum (0.9%) and Tri-chostrongylus species (0.9%). The prevalence of co-infection of malaria parasite and intestinal helminths was 4.3%. The mean malaria parasite density in the study was 173 ± 335 p/μl while the mean egg count of A. lumbricoides (1345 ± 3137 egg/gms) was the highest for intestinal helminth. Ages 4-7 years had the highest prevalence (31%) and mean parasite density (109.3 p/μl) of malaria. The distribution of mean parasite density of malaria shows a gradual decrease as the age increases. For intestinal helminth, the older age group (12-15 years) was generally more infected with A. lumbricoides recording the highest prevalence of 46.5%. The highest intensity (egg output) of 2040 egg/gm of stool was also recorded in A. lumbricoides among age group 8-11 years. Age group 12-15 years had the highest prevalence of malaria and intestinal helminth co-infection (9.3%).
Table 1.
Prevalence of malaria parasite and intensity of intestinal helminth by age
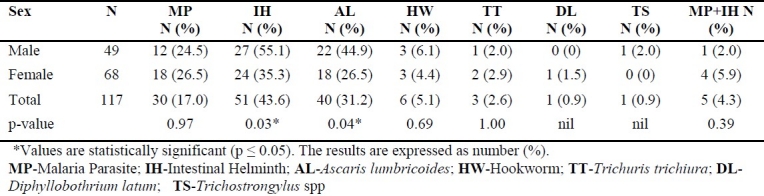
The prevalence of malaria parasite and intestinal helminths by sex is shown in table 2. Females had a higher prevalence (26.5%) of malaria compared to their male counterparts (24.5%) but the difference was not statistically significant. Males tend to have a higher prevalence with intestinal helminths (55.1%) compared to their female counterparts (35.3%) and the difference was statistically significant (p = 0.0390). For A. lumbricoides, males (44.9%) were more infected compared to females (26.5%) and the difference was statistically significant (p = 0.0487). For the co-infection of both malaria and any of the intestinal helminths, females (5.9%) were more infected than males (2.0%) showing no statistically significant difference.
Table 2.
Prevalence of malaria parasite and intestinal helminth by sex
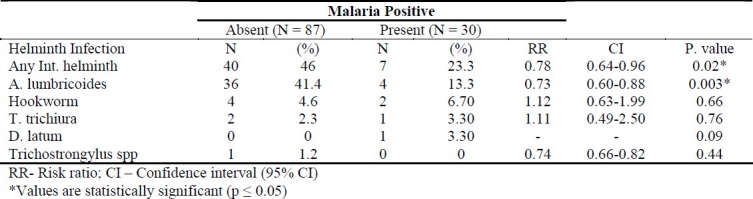
The association between malaria parasite and intestinal helminths among the study subjects is shown in Table 3. Out of the 30 (25.6%) children that were positive for malaria, 23 (77%) were co-infected with intestinal helminths and the difference was statistically significant (p = 0.0053). Children who were infected with intestinal helminth were almost approximately one time (RR = 0.7295) as likely to be infected with P. falciparum as children with no worm infection.
Table 3.
Associations between malaria parasite and intestinal helminth infection among the study subjects.
Discussions
The results of the present study showed the occurrence of asymptomatic falciparum malaria and intestinal helminths parasites of public health importance among schoolchildren in primary schools in Kajola, Osun state, Nigeria. The high prevalence of asymptomatic malaria (25.6%) recorded in the study confirms previous reports that malaria is hyperendemic in Osun State Nigeria.22 It was previously observed that the majority of malaria infections in individuals living in endemic regions are asymptomatic with the young children bearing the highest burden of disease and carrying asymptomatic infections for most of the time.28,29 The decline of P. falciparum parasite density with age, observed in this study is characteristic of both symptomatic and asymptomatic infection in endemic regions.30–32 The mean values observed are lower compared to asymp-tomatic values found in a longitudinal survey of malaria infection in individuals living in this area22 and other highly malarious endemic region of Papua New.30,33
Like malaria, soil transmitted helminths are highly prevalent in rural communities as a result of poor sanitary conditions prevailing in most of these areas. This study recorded a prevalence of 43.6% for helminth infections, with A. lumbricoides having the highest prevalence, followed in decreasing order by hookworm and T. trichiura infections. In earlier studies among school children in Osun State Nigeria, the prevalence of these 3 species were in similar order but these works recorded higher prevalence.34–35 The lower prevalence recorded in this study might reflect the increase awareness and improved sanitary conditions brought about by urbanization. While the previous studies were conducted in typical rural communities, the present study was conducted in semi-urban community that shares proximity with Osogbo the state capital of Osun State Nigeria. A significant difference has previously been reported in infection rate between urban and rural school children in this area.36
In the present study, the co-infection of malaria and intestinal helminths is 4.3% and of the children who had malaria, > 23% also had some types of worm infections. The geo-helminth-positive children tended to be parasitaemic and there was statistically significant association between helminth status and parasitaemia. A similar observation was made in Thailand, where, a positive and statistically significant association between geohelminth and malarial infection was also reported.25 In this study, it was observed that children with A. lumbricoides infections were almost two times as likely to have P. falciparum infection as children without A. lumbricoides infection. The mechanism behind this association is not clearly understood, but could be that Th2 profile-associated immunoglobulin E production seen in Ascaris infection may down-modulate Th1 antimalarial immune responses, resulting in increased risk of malaria infection.37 There are, however, conflicting reports on how helminth infection affects malarial infection and vice versa. A similar association was previously observed among cohort of pregnant women in Ghana. In Uganda, geohelminth positive children tended to be parasitaemic but were not statistically significant.38
In developing countries, although mild anaemia occurs commonly among the general population, moderately severe anaemia is most frequently seen in areas where infections can cause or exacerbate anaemia.39 In this study, the presence of both malaria and helminths parasites among children was not associated with anaemia. This observation could be attributed to the fact that none of the children in this study had a high density of both P. falciparum and helminths infection thus, these parasites are unlikely to have contributed significantly to the presence of anemia in this study population. In individuals with precarious iron balance, however, relatively small hookworm loads may result in anemia. Also, high helminths burden in co-infections involving P. falciparum may in the long run exacerbate anemia caused by the malaria parasites.
In conclusion, this study observed co-infection of both P. falciparum and intestinal helminths among school children in Osun State Nigeria. Given the plausible hypothesis of a biological interaction between helminths and P. falciparum and increasing advocacy for deworming, there is a need for prospective studies of the effects of helminths and the treatment of helminth infection on P. falciparum in this area. This work is undoubtedly the first study to document the prevalence of asymptomatic malaria among children in this area. The success of malaria control will depend on a systematic understanding of the micro-geographic risk of malaria transmission that would enable identification of high risk spots. The identification, quantification, and spatiotemporal mapping of the asymptomatic subpopulation will provide an opportunity to estimate the epidemiological importance of this group to malaria transmission.
Authors’ Contributions
OO carried out the design and coordinated the study and also prepared the manuscript, participated in most of the experiments and prepared the manuscript. AMA, AAA and OAA were involved in sample collection and laboratory methods. OSB provided assistance in the field and laboratory aspects. OAA supervised the study. All authors have read and approved the content of the manuscript.
Acknowledgments
The authors are grateful to all parents and guardian who volunteered to participate in the study.
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64(1-2 Suppl):1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- 2.Bereczky S, Liljander A, Rooth I, Faraja L, Granath F, Montgomery SM, et al. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 2007;9(1):103–10. doi: 10.1016/j.micinf.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Mwangi TW, Fegan G, Williams TN, Kinyanjui SM, Snow RW, Marsh K. Evidence for over-dispersion in the distribution of clinical malaria episodes in children. PLoS One. 2008;3(5):e2196. doi: 10.1371/journal.pone.0002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp.as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42(5):777–9. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- 6.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird JK. Age-dependent characteristics of protection v.susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol. 1998;92(4):367–90. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- 8.Bruce-Chwatt LJ. Malaria in African infants and children in Southern Nigeria. Ann Trop Med Parasitol. 1952;46(2):173–200. doi: 10.1080/00034983.1952.11685522. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein JL, Schleinitz MD, Carabin H, McGarvey ST. Decision-model estimation of the age-specific disability weight for schistosomiasis japonica: a systematic review of the literature. PLoS Negl Trop Dis. 2008;2(3):e158. doi: 10.1371/journal.pntd.0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Hesran JY, Akiana J, Ndiaye el HM, Dia M, Senghor P, Konate L. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans R Soc Trop Med Hyg. 2004;98(7):397–9. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Vannaphan S, Traore B, et al. Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am J Trop Med Hyg. 2001;65(6):834–6. doi: 10.4269/ajtmh.2001.65.834. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2003;97(2):198–9. doi: 10.1016/s0035-9203(03)90117-9. [DOI] [PubMed] [Google Scholar]
- 13.Nkuo-Akenji TK, Chi PC, Cho JF, Ndamukong KK, Sumbele I. Malaria and helminth co-infection in children living in a malaria endemic setting of mount Cameroon and predictors of anemia. J Parasitol. 2006;92(6):1191–5. doi: 10.1645/GE-895R.1. [DOI] [PubMed] [Google Scholar]
- 14.Scolari C, Torti C, Beltrame A, Matteelli A, Castelli F, Gulletta M, et al. Prevalence and distribution of soil-transmitted helminth (STH) infections in urban and indigenous schoolchildren in Ortigueira, State of Parana, Brasil: implications for control. Trop Med Int Health. 2000;5(4):302–7. doi: 10.1046/j.1365-3156.2000.00549.x. [DOI] [PubMed] [Google Scholar]
- 15.Ekvall H, Premji Z, Bennett S, Bjorkman A. Hemoglobin concentration in children in a malaria holoendemic area is determined by cumulated Plasmodium falciparum parasite densities. Am J Trop Med Hyg. 2001;64(1-2):58–66. doi: 10.4269/ajtmh.2001.64.58. [DOI] [PubMed] [Google Scholar]
- 16.Ogungbamigbe TO, Ojurongbe OO, Ogunro PS, Olowe OA, Elemile PO. Prevalence and transmission pattern of Plasmodium falciparum infection in Osogbo metropolis, southwest, Nigeria. Afr J Med Med Sci. 2007;36(4):305–10. [PubMed] [Google Scholar]
- 17.Ekpo UF, Odoemene SN, Mafiana CF, Sam-Wobo SO. Helminthiasis and hygiene conditions of schools in Ikenne, Ogun State, Nigeria. PLoS Negl Trop Dis. 2008;2(1):e146. doi: 10.1371/journal.pntd.0000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojurongbe O, Awe F, Olowe O, Okanlawon B, Adeyeba O. Prevalence of Soil Transmitted Helminth Infections in a Tertiary Institution in Western Nigeria. New York Science Journal. 2010;3:1–5. [Google Scholar]
- 19.Adeoye G, Osayemi C, Oteniya O, Onyemekeihia S. Epidemiological studies of intestinal helminthes and malaria among children in Lagos, Nigeria. Pak J Biol Sci. 2007;10:2208–12. doi: 10.3923/pjbs.2007.2208.2212. [DOI] [PubMed] [Google Scholar]
- 20.Adefemi S, Musa O. Intestinal Helminthes Infestation Among Pupils in Rural and Urban Communities of Kwara State, Nigeria. African Journal of Clinical and Exprimental Microbiology. 2006;7(3):208–11. [Google Scholar]
- 21.Ibidapo C, Okwa O. The Prevalence and Intensity of Soil Transmitted Helminths in a Rural Community, Lagos Suburb, South West Nigeria. International Journal of Agriculture and Biology. 2008;10:89–92. [Google Scholar]
- 22.Ojurongbe O, Ogungbamigbe TO, Fagbenro-Beyioku AF, Fendel R, Kremsner PG, Kun JF. Rapid detection of Pfcrt and Pfmdr1 mutations in Plasmodium falciparum isolates by FRET and in vivo response to chloroquine among children from Osogbo, Nigeria. Malar J. 2007;6:41. doi: 10.1186/1475-2875-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg. 2005;73(6):1124–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Mutapi F, Roussilhon C, Mduluza T, Druilhe P. Anti-malaria humoral responses in children exposed to Plasmodium falciparum and Schistosoma haematobium. Mem Inst Oswaldo Cruz. 2007;102(3):405–9. doi: 10.1590/s0074-02762007005000046. [DOI] [PubMed] [Google Scholar]
- 25.Nacher M, Singhasivanon P, Treeprasertsuk S, Vannaphan S, Traore B, Looareesuwan S, et al. Intestinal helminths and malnutrition are independently associated with protection from cerebral malaria in Thailand. Ann Trop Med Parasitol. 2002;96(1):5–13. doi: 10.1179/000349802125000448. [DOI] [PubMed] [Google Scholar]
- 26.Cheesbrough M, editor. United Kingdom: Cambridge University; 1998. District laboratory practice in tropical countries. Part I. [Google Scholar]
- 27.Idris M, Al-Jabri AM. Usefulness of Kato-Katz and trichrome staining as diagnostic methods for parasitic infections in clinical laboratories. SQU Journal for Scientific Research: Medical Sciences. 2001;3(2):65–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood BM, Groenendaal F, Bradley AK, Clarke SE, Magnussen P, Olsen A, et al. Ethnic differences in the prevalence of splenomegaly and malaria in The Gambia. Ann Trop Med Parasitol. 1987;81(4):345–54. doi: 10.1080/00034983.1987.11812130. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood BM. Asymptomatic malaria infections--do they matter? Parasitol Today. 1987;3(7):206–14. doi: 10.1016/0169-4758(87)90061-5. [DOI] [PubMed] [Google Scholar]
- 30.Bruce MC, Donnelly CA, Packer M, Lagog M, Gibson N, Narara A, et al. Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology. 2000;121(Pt 3):247–56. doi: 10.1017/s0031182099006344. [DOI] [PubMed] [Google Scholar]
- 31.Smith T, Hurt N, Teuscher T, Tanner M. Is fever a good sign for clinical malaria in surveys of endemic communities? Am J Trop Med Hyg. 1995;52(4):306–10. doi: 10.4269/ajtmh.1995.52.306. [DOI] [PubMed] [Google Scholar]
- 32.Rogier C, Commenges D, Trape JF. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg. 1996;54(6):613–9. doi: 10.4269/ajtmh.1996.54.613. [DOI] [PubMed] [Google Scholar]
- 33.Cox MJ, Kum DE, Tavul L, Narara A, Raiko A, Baisor M, et al. Dynamics of malaria parasitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans R Soc Trop Med Hyg. 1994;88(2):191–7. doi: 10.1016/0035-9203(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 34.Ijagbone I, Olagunju T. Intestinal helminth parasites in school children in Iragbiji, boripe local government, Osun state, Nigeria. African Journal of Biomedical Research. 2006;9(1):63–6. [Google Scholar]
- 35.Kirwan P, Asaolu SO, Molloy SF, Abiona TC, Jackson AL, Holland CV. Patterns of soil-transmitted helminth infection and impact of four-monthly albendazole treatments in preschool children from semi-urban communities in Nigeria: a double-blind placebo-controlled randomised trial. BMC Infect Dis. 2009;9:20. doi: 10.1186/1471-2334-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oninla SO, Owa JA, Onayade AA, Taiwo O. Intestinal helminthiases among rural and urban schoolchildren in south-western Nigeria. Ann Trop Med Parasitol. 2007;101(8):705–13. doi: 10.1179/136485907X241406. [DOI] [PubMed] [Google Scholar]
- 37.Yatich NJ, Yi J, Agbenyega T, Greenwood AM, Shenton F, Tulloch S, et al. Malaria and intestinal helminth co-infection among pregnant women in Ghana: prevalence and risk factors. Am J Trop Med Hyg. 2009;80(6):896–901. [PubMed] [Google Scholar]
- 38.Shapiro AE, Tukahebwa EM, Kasten J, Clarke SE, Magnussen P, Olsen A, et al. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans R Soc Trop Med Hyg. 2005;99(1):18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 39.van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr. 2000;72(1 Suppl):247–56. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]


