Abstract
Background:
The evaluation and management of various hepatic lesions is a common clinical problem and their appropriate clinical management depends on accurate diagnoses.
Aims:
To study the cytomorphological features of distinctive non-neoplastic and neoplastic lesions of the liver and to evaluate the sensitivity, specificity and diagnostic accuracy of ultrasonography (USG)-guided fine needle aspiration cytology (FNAC) in the diagnosis of liver diseases.
Materials and Methods:
Seventy-two patients with evidence of liver diseases underwent USG-guided, percutaneous FNAC. Cytomorphological diagnoses were correlated with clinical, biochemical and radiological findings, histopathological diagnoses and follow-up information.
Results:
The age of the patients ranged from eight months to 90 years with 48 males (66.67%) and 24 females (33.33%). Of the 72 cases, the cytological diagnosis was rendered in 71 patients and smears were inadequate for interpretation in one case. Neoplastic lesions (68.06%) were more common than non-neoplastic lesions (30.56%). The majority of the neoplastic lesions were hepatocellular carcinomas (36.12%) followed by metastatic adenocarcinomas (19.45%). Among non-neoplastic lesions, cirrhosis was the commonest lesion (8.34%). The overall diagnostic accuracy of FNAC was 97.82% with a sensitivity and specificity of 96.87 and 100% respectively.
Conclusion:
USG-guided FNAC of the liver is a safe, simple, cost-effective and accurate method for cytological diagnosis of hepatic diffuse, focal/nodular and cystic lesions with good sensitivity and specificity.
Keywords: Fine needle aspiration cytology, hepatic lesions, ultrasonography
Introduction
The liver is involved by many non-neoplastic and neoplastic diseases. Evaluation and management of hepatic lesions is a common clinical problem and their appropriate clinical management depends on accurate diagnosis.[1] The differential diagnosis of hepatic mass lesions includes primary liver tumors (benign or malignant), metastatic deposits, congenital and acquired cysts, abscesses and granulomas.[2] Ultrasonography (USG) or computed tomography (CT)-guided fine needle aspiration cytology (FNAC) is an accurate method for arriving at a definite tissue diagnosis in focal liver lesions.[3] Occasionally, inflammatory lesions or diffuse liver diseases may mimic mass-like lesions or appear as non-homogeneous regions on radiographs. Such lesions also are sampled by FNAC to rule out neoplasms from differential diagnosis.[4]
The aim of the present study was to describe the cytomorphological features of various liver lesions with histopathological correlation and to evaluate the diagnostic accuracy of USG-guided FNAC in the diagnosis of liver diseases.
Materials and Methods
Seventy-two patients with clinical, biochemical and radiological evidence of liver diseases with normal prothrombin time were subjected to USG-guided FNAC over a two-year period prospectively. The patients with hemangioma and hydatid disease of liver diagnosed by USG were excluded to prevent undue complications. The cytological material was obtained using 20 or 22-gauge, 90-mm spinal needle introduced into the lesion under ultrasound evaluation. The smears were stained by May-Grόnwald-Giemsa, Papanicolaou, hematoxylin and eosin (H and E) stains. Reticulin, periodic acid-Schiff (PAS), Gram's and Ziehl-Neelson (ZN) stains were done whenever needed. The specimen for histopathology (46/72) was obtained by core needle biopsy performed during the FNA using Vim-Silverman liver biopsy needle. Visible tissue fragments whenever obtained during FNA were studied as cell blocks. Cyto-histo-morphological diagnoses were correlated and the specificity, sensitivity and accuracy of FNAC were evaluated.
Results
Patients’ age ranged from eight months to 90 years out of which 48 were males (66.67%) and 24 (33.33%) were females. USG liver revealed solitary mass in 37 cases (51.38%), multifocal lesions in 26 (36.12%), diffuse parenchymal disease in eight (11.12%) and normal echogenicity in one (1.38%). Cytomorphologically, liver lesions were categorized into: (1) non-neoplastic lesions (2) neoplastic lesions and (3) inadequate for interpretation [Table 1]. Diagnosis was rendered in 71/72 cases. Aspirate was inadequate for interpretation in one case.
Table 1.
FNAC diagnoses in 72 patients
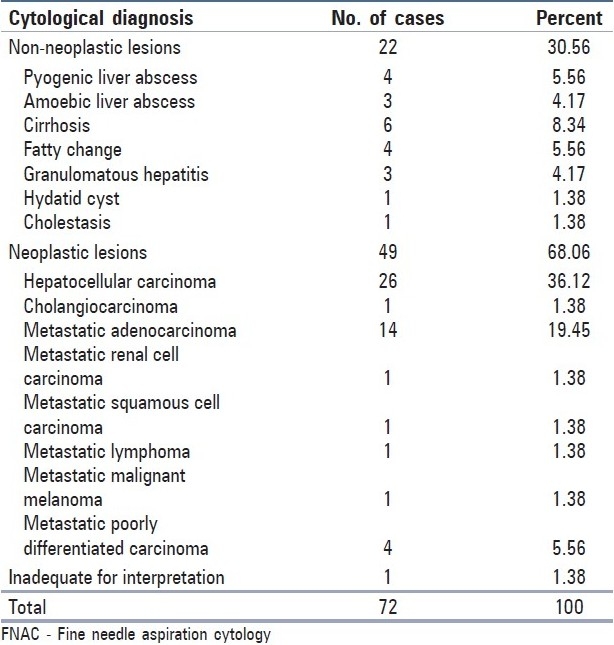
Non-neoplastic lesions included pyogenic liver abscess, amoebic liver abscess, cirrhosis, and fatty liver and granulomatous hepatitis. The smears from pyogenic liver abscess predominantly showed neutrophils [Figure 1a]. Aspirate from amoebic liver abscess yielded orange-brown colored fluid and the smears showed trophozoites of Entamoeba histolytica [Figure 1b].
Figure 1.
FNAC of liver: (a) Pyogenic liver abscess - polymorphonuclear leucocytes, necrotic cells and debris (MGG, ×100). (b) Amoebic liver abscess - Trophozoite of E. histolytica (arrow) on a necrotic background with inflammatory cells (MGG, ×400). (c) Cirrhosis - Reactive hepatocytes with degenerative/ regenerative features; cluster of bile ductal cells; fragment of fibrous tissue with spindle - shaped nuclei (MGG, ×100). Inset: Reactive hepatocytes adjacent to a thick fragment of fibrous tissue (MGG,×400). (d) Granulomatous hepatitis - Aggregate of epithelioid histiocytes and Langhan's giant cell (H and E, ×400)
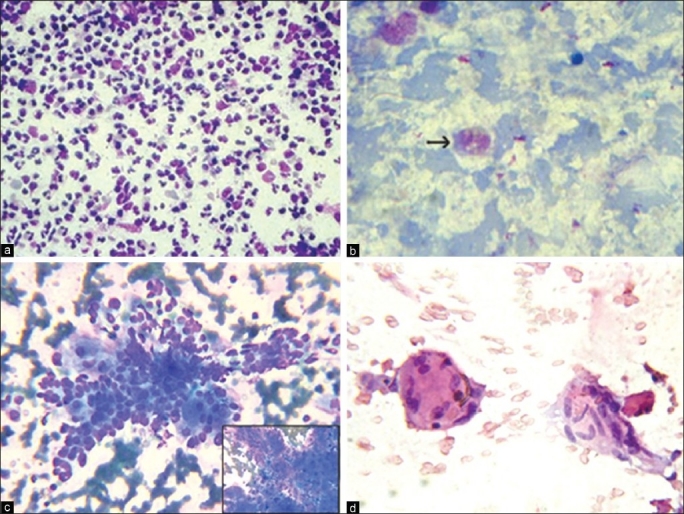
Among six cases of cirrhosis [Figure 1c], five were confirmed histopathologically and one turned out to be hepatocellular carcinoma (HCC) on tissue section. We diagnosed fatty liver in four cases, three had diffuse fatty change and focal fatty change (FFC) was reported in one female patient. The smears from granulomatous hepatitis showed epithelioid histiocytes in singles and small clusters along with foreign body and Langhan's type of multinucleated giant cells and lymphocytes [Figure 1d]. One patient was diagnosed as having pulmonary tuberculosis. In two patients there was no clinical, radiological and laboratory evidence of other granulomatous diseases. Aspirate from hydatid cyst (radiologically diagnosed as pyogenic liver abscess) yielded thick, turbid fluid which showed refractile dagger-shaped hooklets and fragments of laminated membrane along with degenerating hepatocytes and mixed inflammatory cells [Figure 2a]. No complications were encountered while aspirating hydatid cyst.
Figure 2.
FNAC of liver: (a) Hydatid cyst liver - Debris and altered blood along with many refractile hooklets of E. granulosus (arrows) (MGG, ×100). (b) WDHCC - Pseudo - acinar formation, intracytoplasmic eosinophilic inclusions and bile (MGG, ×400). Inset: Intra - nuclear inclusion (arrow) (Pap, ×400). (c) MDHCC - Discohesive pleomorphic hepatocytes centrally transgressed by a proliferating band of endothelium (Pap, ×400). (d) PDHCC - Discohesive malignant cell clusters with poorly preserved hepatocytic features, extremely high N:C ratio (H and E, ×400)
No benign neoplasms were reported in our study. HCC was the most common neoplastic liver lesion (36.12%), 18 were males (69.23%) and eight were females (30.76%). USG showed 22 solitary and four multifocal lesions. The largest and smallest lesion measured 18 × 18 cm and 6 × 4 cm respectively. Serum alfa-feto protein levels were available in 15 patients, nine of them had high values. Hepatitis B surface antigen (HBsAg) was positive in four patients.
Cytologically, HCC was classified into well, moderately and poorly differentiated types (W-, M- and P-HCC) which accounted for nine (34.6%), 12 (46.1%) and five (19.2%) cases respectively. Sixteen cases were histopathologically correlated. The main cytological features in W-HCC were hypercellularity with broad trabeculae, endothelial rimming/transgression of vessels in the cell clusters, bare atypical nuclei, large polygonal cells with abundant eosinophilic granular cytoplasm, intracytoplasmic bile, increased nucleus to cytoplasm (N:C) ratio, central round nucleus and intranuclear inclusions [Figure 2b]. M-HCC had many features of W-HCC. Endothelial rimming or transgressing of cell clusters, eccentric nuclei, multinucleation, multiple nucleoli and macronucleoli were more associated with M-HCC [Figure 2c]. P-HCC showed cells in sheets, small groups and singles. Anisocytosis, anisonucleosis, irregular nuclear chromatin, hyperchromasia, multiple nuclei, macronucleoli and bare atypical nuclei were seen in all the patients [Figure 2d]. Transgressing endothelium, inflammation, necrosis and giant cells were seen in 40% of cases. Multinucleated giant cells with three or more atypical nuclei were seen in all grades of HCC.
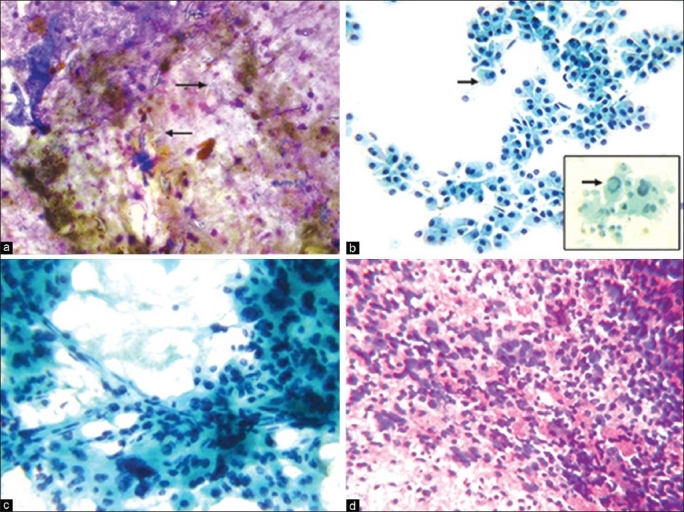
Metastatic tumors constituted 30.56% and adenocarcinoma was the commonest type (19.45%). Primary sites of adenocarcinoma were: Colon and rectum (5), ovary (2), breast (2), lung (1) and unknown primary (4). The smears revealed hypercellularity with columnar to cuboidal cells arranged in monolayered sheets, palisade forms, acinar pattern and in singles having vacuolated or granular and eosinophilic cytoplasm. The cells showed altered N: C ratio, anisonucleosis with central or eccentrically placed nucleus and fine-coarse dispersed chromatin. Many showed benign hepatocytes in the background. Inflammation, necrosis and fibrosis were prominent in some cases. We observed one case each of metastatic renal cell carcinoma (RCC) [Figure 3a] and malignant melanoma [Figure 3b]. In metastatic squamous cell carcinoma (SCC), smears showed squamoid, tadpole-like and spindle-shaped cells with well-defined abundant keratinized cytoplasm and pleomorphic and hyperchromatic nuclei. Final diagnosis of cytologically diagnosed poorly differentiated carcinomas could not be evaluated as the patients were referred to a cancer hospital and were lost to follow-up.
Figure 3.
FNAC of liver: (a) Metastatic renal cell carcinoma - Tissue fragments with discohesive cells adhering to strands of pink stroma/basement membrane; enlarged pleomorphic nuclei; variable N:C ratio; abundant vacuolated cytoplasm (MGG, ×400). (b) Metastatic malignant melanoma - Pleomorphic cells with well - defined cytoplasm; eccentric nuclei; multinucleated cells; prominent nucleoli; dust - like pigment within the cytoplasm, macrophages in the background (MGG, ×400)
Histopathology was available for correlation of cytological diagnoses in 46/72 cases. In 14 cases, cytological diagnoses of non-neoplastic diseases were concordant with histopathology. One discordant case (cytologically diagnosed as cirrhosis) was histopathologically confirmed as HCC. We could accurately diagnose all malignant neoplasms cytologically. In all 31 cases, the cytological diagnoses of malignant neoplasms were concordant with histopathology, but two metastatic poorly differentiated carcinomas were cytologically misdiagnosed as P-HCC. The sensitivity and specificity of USG-guided FNAC in the diagnosis of liver diseases was 96.87 and 100% respectively, with a positive predictive value of 100% and negative predictive value of 93.34%, and overall diagnostic accuracy of 97.82%.
Discussion
USG-guided FNAC offers accuracy without major complications and minimal intervention at less cost.[5] Although imaging techniques have helped greatly with the early and accurate diagnosis of liver abscess, the appearances are often non-specific. There is some overlap between the radiologic features of liver abscesses, HCC and metastases. Tumors, primary or secondary, may undergo extensive necrosis, with the resultant radiologic image of the cavitary neoplasms mimicking abscesses; abscesses are accompanied by proliferative reactive changes, making radiologic differentiation from a neoplastic process almost impossible. In these situations FNAC plays an essential complementary role.[6]
In non-pyogenic abscesses, further investigation for amoebae should be carried out if the cytological examination reveals much necrotic debris notably lacking neutrophils. Amoebae are unlikely to be detected in the smears unless tissue from the walls of the abscess cavity is examined.[6] However, trophozoites of E. histolytica were evident in our case. Amoebic liver abscess can also be diagnosed by the presence of serum anti-amoebic antibodies.[6,7]
The presence of hepatocytes entangled within fibrous strands, increased numbers of bile duct epithelium, Kupffer cells, dysplastic and mildly pleomorphic hepatocytes favors diagnosis of cirrhosis.[8] The presence of fibrous strands, intranuclear vacuoles and large nucleoli and absence of characteristic transgressing and peripheral endothelium favored the cytological diagnosis of cirrhosis in one case, but was concluded as HCC on tissue section.
The features in fatty liver were consistent with the description of Leiman[2] and Tao.[9] Hepatic steatosis is the most common finding in liver pathology and it generally involves the entire liver but may produce a circumscribed, nodular lesion first described as FFC in the 1980s.[10] Zeppa et al.,[11] reported two cases of FFC.
The diagnostic value of cytological findings indicating granulomatous disease of the liver may be of variable clinical value and significance since granulomas are found in a wide variety of conditions. Indian studies have shown that 68% of granulomas in liver biopsies are of tuberculous etiology.[8,12]
Liver FNAC is used mainly for diagnosing hepatic malignancies, primary or metastatic.[13]
We have made an attempt to classify HCC into W-HCC, M-HCC and P-HCC based on the features described by Bottles et al.,[13] and Pitman et al.,[14] Table 2 compares the cytomorphological features of HCC with the Wee et al.,[15] study.
Table 2.
Comparison of cytological features of HCC
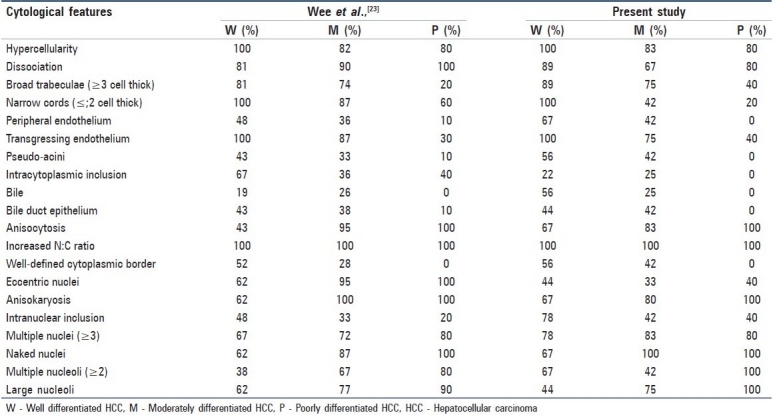
For the majority of hepatic masses, the cytodiagnosis and categorization of HCC into three grades pose no problems. Hypercellular aspirates composed of cohesive clusters of atypical hepatocytes with arborescent, tongue-like projections of broad trabeculae, with or without peripheral endothelial rimming are pathognomonic of classic HCC. As the tumor grade increases, there is corresponding increase in cellular dissociation, with less evidence of transgressing and peripheral endothelium and fewer trabeculae. Peripheral endothelial rimming is observed less frequently than transgressing endothelium in the broad trabeculae.[15]
Intracytoplasmic eosinophilic inclusions were present in 22-25% HCCs. Though the presence of intracytoplasmic inclusions strongly supports HCC, they have also been reported in ovarian, breast, lung and adrenal gland tumors and in asbestosis lung.[16] The presence of intracytoplasmic bile is a well-established diagnostic feature of HCC.Anisocytosis and anisonucleosis were prominent features (>80%) in M-HCC and P-HCCs. Increased N:C ratio was the single most useful parameter for identifying the malignant hepatocytes. The frequency of eccentric nuclei, irregular nuclear contours and increased chromatin density increases with higher grades of HCC. Intranuclear inclusions due to invagination of cytoplasm into the nucleus were evident in all the groups, with a maximum frequency in WD-HCC. Increased frequency of multiple nucleoli and macronucleoli are seen as the grades of HCCs increase.[15] Atypical hepatocytic naked nuclei were seen in increasing numbers with increase in grades of HCC, which distinguishes highly W-HCC from benign lesions.[17]
Difficulty in cytological diagnosis of HCC arises at the ends of the spectrum—distinguishing W-HCC from benign lesions and separating less-differentiated HCC from metastatic malignancies or other tumors.[1,18] A stepwise logistic regression analysis has been used to distinguish HCC and reactive liver cells[19] and between HCC and metastatic tumor cells.[13] The most useful criteria to separate highly W-HCC cells from reactive liver cells are: Architectural features on the smears/cell block sections, hypercellularity; arborescent, cohesive clusters; broad trabeculae; transgressing/peripheral endothelium; small, monotonous hepatocytes with nuclear crowding, increased N:C ratio, cytoplasmic hyaline inclusions, atypical naked nuclei and tumor giant cells. Well-defined cytoplasmic borders, abundant thick and monotonous cytoplasm, eccentric nuclei, thick nuclear membranes, irregular nuclear contours, increased chromatin density, irregular chromatin distribution and macronucleoli were not always detectable in highly W-HCC. In fact, some of them were seen in dysplastic hepatocytes.[18,20]
Three criteria differentiate HCC from metastatic tumor: Polygonal cells with centrally placed nuclei, malignant cells separated by sinusoidal capillaries and bile. Two additional criteria, namely, endothelial cells surrounding tumor cell clusters and intranuclear inclusions were identified as being important secondary criteria for HCC.[13] However, in our study, two metastatic malignancies reported as P-HCC on FNAC, were histopathologically identified as poorly differentiated carcinomas. The presence of transgressing endothelium and intranuclear inclusions misled the diagnosis of P-HCC in these cases.
Although primary carcinomas may be poorly differentiated the cytological features which favoured metastatic poorly differentiated carcinoma were the presence of benign hepatocytes, necrosis along with irregular clusters and dissociated malignant cells. Immunohistochemistry might be of value in differentiating P-HCC from other poorly differentiated tumors.
The frequency of metastatic liver lesions was lower than the frequencies reported by other studies.[3,21] Adenocarcinoma is the most common metastatic malignancy[3] and colonic adenocarcinoma is the commonest primary source for liver metastasis.[2] Pinto et al.,[21] observed two cases of metastatic RCC. Cytological features of metastatic SCC were similar to those described by Kuo et al.[1]
The diagnostic accuracy of FNAC of liver in our study is on par with other series [Kuo et al.,[1] (86.1%), Ramdas et al.,[22] (87.5%), Cochand-Priollet et al.,[23] (82.6%) and Franca et al.,[24] (78%)]. With an accuracy of 97.82% for liver lesions, FNAC is a valuable method that allows rapid diagnosis.
Conclusion
USG-guided FNAC is very useful in the diagnosis of hepatic lesions as it is a quick, safe, simple, cost-effective and accurate method. Early diagnosis by guided aspiration minimizes further ancillary investigations and decreases the length of hospital stay. FNAC can accurately distinguish non-neoplastic from neoplastic lesions, categorize different non-noeplastic lesions and differentiate primary from metastatic tumors, which is helpful for the management of hepatic lesions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kuo FY, Chen WJ, Lu SN, Wang JH, Eng HL. Fine needle aspiration cytodiagnosis of liver tumors. Acta Cytol. 2004;48:142–8. doi: 10.1159/000326307. [DOI] [PubMed] [Google Scholar]
- 2.Leiman G. Liver and Spleen. In: Orell SR, Sterret GF, Whitaker D, editors. Fine needle aspiration cytology. 4th ed. New Delhi: Churchill Livingstone; 2005. pp. 293–316. [Google Scholar]
- 3.Das DK, Tripathi RP, Chachra KL, Sodhani P, Parkash S, Bhambhani S. Role of guided fine needle aspiration cytology in diagnosis and classification of liver malignancies. Trop Gastroenterol. 1997;18:101–6. [PubMed] [Google Scholar]
- 4.Tsui WM, Cheng F, Lee Y. Fine needle aspiration cytology of liver tumors. Ann Contemp Diagn Pathol. 1998;2:79–93. [Google Scholar]
- 5.Rasania A, Pandey CL, Joshi N. Evaluation of FNAC in diagnosis of hepatic lesion. J Cytol. 2007;24:51–4. [Google Scholar]
- 6.Wee A, Nilsson B, Yap I, Chong SM. Aspiration cytology of liver abscess.With an emphasis on diagnostic pitfalls. Acta Cytol. 1995;39:453–62. [PubMed] [Google Scholar]
- 7.Roy M, Bhattacharyya A, Dasgupta S, Sanyal S. Fallacies of fine needle aspiration cytology surgical lesion of the liver. J Indian Med Assoc. 1994;92:285–7. [PubMed] [Google Scholar]
- 8.Gatphoh ED, Gaytri S, Babina S, Singh AM. Fine needle aspiration cytology of liver: a study of 202 cases. Indian J Med Sci. 2003;57:22–5. [PubMed] [Google Scholar]
- 9.Tao LC. Liver and pancreas. In: Bibbo M, editor. Comprehensive cytopathology. 2nd ed. Philadelphia: WB Saunders Company; 1997. pp. 827–63. [Google Scholar]
- 10.Pagani JJ. Biopsy of focal hepatic lesions.Comparison of 18 and 22 gauge needles. Radiology. 1983;147:673–5. doi: 10.1148/radiology.147.3.6844603. [DOI] [PubMed] [Google Scholar]
- 11.Zeppa P, Anniciello A, Vetrani A, Palombini L. Fine needle aspiration biopsy hepatic focal fatty change.A report of two cases. Acta Cytol. 2002;46:567–70. doi: 10.1159/000326879. [DOI] [PubMed] [Google Scholar]
- 12.Radhika S, Rajawanshi A, Kochhar R, Kochhar S, Dey P, Roy P. Abdominal tuberculosis.Diagnosis by fine needle aspiration cytology. Acta Cytol. 1993;37:673–8. [PubMed] [Google Scholar]
- 13.Bottles K, Cohen MB, Holly EA, Chiu SH, Abele JS, Cello JP, et al. A step-wise logistic regression analysis of hepatocellular carcinoma-An aspiration biopsy study. Cancer. 1988;62:558–63. doi: 10.1002/1097-0142(19880801)62:3<558::aid-cncr2820620320>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Pitman MB, Szyfelbein WM. In: Fine needle aspiration biopsy of liver. Boston: Butterworth-Heinemann; 1994. Primary malignant liver tumors; pp. 47–86. [Google Scholar]
- 15.Wee A, Nilsson B, Tan LK, Yap I. Fine needle aspiration biopsy of hepatocellular carcinoma.Diagnostic dilemma at the ends of the spectrum. Acta Cytol. 1994;38:347–54. [PubMed] [Google Scholar]
- 16.MacDonld K, Bedard YC. Cytologic, ultrastructural and immunologic features of intracytoplasmic hyaline bodies in fine needle aspiration biopsy of hepatocellular carcinoma. Acta Cytol. 1990;34:197–200. [PubMed] [Google Scholar]
- 17.Pedio G, Landolt L, Zöbeli L, Gut D. Fine needle aspiration of the liver.Significance of hepatocytic naked nuclei in the diagnosis of hepatocellular carcinoma. Acta Cytol. 1988;32:437–42. [PubMed] [Google Scholar]
- 18.Soyuer I, Ekinci C, Kaya M, Genc Y, Bahar K. Diagnosis of hepatocellular carcinoma by fine needle aspiration cytology.Cellular features. Acta Cytol. 2003;47:581–9. doi: 10.1159/000326572. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MB, Haber MM, Holly EA, Ahn DK, Bottles K, Stoloff AC. Cytologic criteria to distinguish hepatocellular carcinoma from nonneoplastic liver. Am J Clin Pathol. 1991;95:125–30. doi: 10.1093/ajcp/95.2.125. [DOI] [PubMed] [Google Scholar]
- 20.Wee A, Nilsson B. Highly well differentiated hepatocellular carcinoma and benign hepatocellular lesions.Can they be distinguished on fine needle aspiration biopsy? Acta Cytol. 2003;47:16–26. doi: 10.1159/000326470. [DOI] [PubMed] [Google Scholar]
- 21.Pinto MM, Avila NA, Heller CI, Criscuolo EM. Fine needle aspiration of the liver. Acta Cytol. 1988;32:15–21. [PubMed] [Google Scholar]
- 22.Ramdas A, Chopra R. Diagnostic accuracy of fine needle aspiration cytology of liver lesions. J Cytol. 2003;20:121–3. [Google Scholar]
- 23.Cochand-Priollet B, Chagnon S, Ferrand J, Blery M, Hoang C, Galian A. Comparison of cytologic examination of smears and histologic examination of tissue cores obtained by fine needle aspiration biopsy of the liver. Acta Cytol. 1987;31:476–80. [PubMed] [Google Scholar]
- 24.Franca AV, Valerio HM, Trevisan M, Escanhoela C, Seva-Pereira T, Zucoloto S, et al. Fine needle aspiration biopsy for improving the diagnostic accuracy of cut needle biopsy of focal liver lesions. Acta Cytol. 2003;47:332–6. doi: 10.1159/000326529. [DOI] [PubMed] [Google Scholar]


