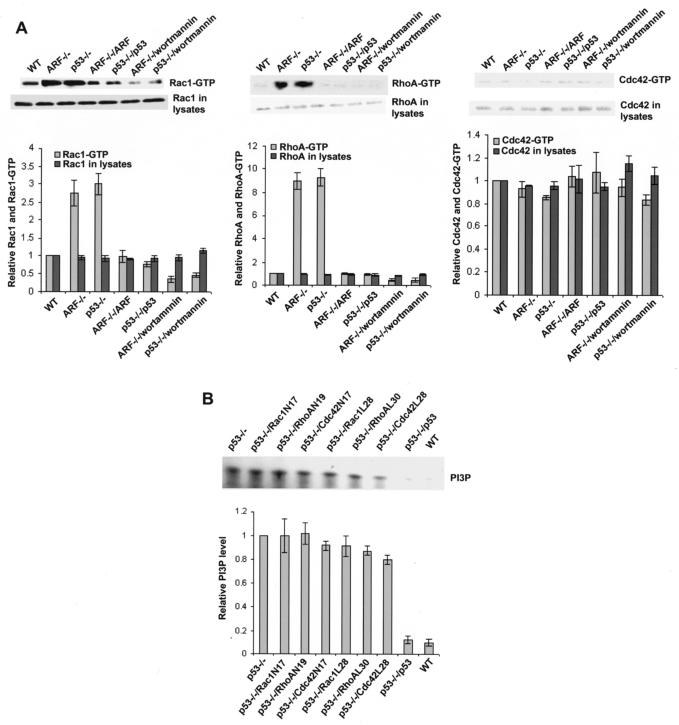
FIG. 1.
Activation of Rac1 and RhoA by the p19Arf or p53 defect is dependent on elevated PI 3-kinase activity. (A) The endogenous Rac1, RhoA, or Cdc42 activities in the Arf or p53 knockout and reconstituted cells with or without wortmannin (50 nM) treatment were assayed by using log phase cells that were serum starved for 12 h. The lysates were subject to GST-PAK1, GST-Rhotekin, or GST-WASP pull-down analysis. The amount of Rac1-GTP, RhoA-GTP, or Cdc42-GTP was detected by Western blotting of the respective glutathione-agarose coprecipitates with anti-Rac1, anti-RhoA, or anti-Cdc42 antibody and was normalized to that of Rac1, RhoA, or Cdc42 in wild-type (WT) MEFs. The results are shown as the means ± the standard deviations of three experiments. (B) The PI 3-kinase activities of the Arf or p53 knockout and reconstituted cells of the log phase cells were assayed. The cells were starved in a medium containing 0.5% serum for 12 h and harvested for anti-p85 immunoprecipitation. The PI 3-kinase activities in the immunoprecipitates were measured by an in vitro lipid kinase assay using exogenous PI as the substrate. The PI3P signals of various MEFs were normalized to those of the p53−/− MEFs in the quantification.