Table 4.
Room-Temperature Asymmetric α-Arylation of Ketones with Chloro- and Bromoarenes Catalyzed by [(R)-BINAP]Ni(η2-NC-Ph)a
 | |||||
|---|---|---|---|---|---|
| 1 |
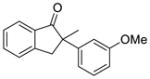 X = Cl (10%) yield: 91%; ee: >99% X = Br (5%) yield: 95%; ee: >99% |
2 |
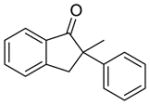 X = Cl (10%) yield: 64%; ee: 98% X = Br (5%) yield: 74%; ee: 99% |
3 |
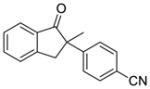 X = Cl (10%) yield: 93%; ee: >99% X = Br (5%) yield: 61%; ee: 97% |
| 4 |
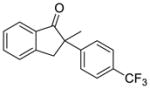 X = Cl (5%) yield: 92%; ee: >99% X= Br (5%) yield: 96%; ee: >99% |
5 |
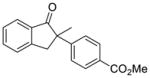 X = Cl (10%) yield: 72%; ee: >99% X = Br (5%) yield: 50%; ee: 98% |
6 |
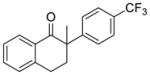 X = Cl (10%) yield: 59%; ee: 95% X = Br (10%) yield: 74%; ee: 83% |
Conditions: Ketones (0.200 mmol), ArX (0.400 mmol), NaOtBu (0.400 mmol), and [(R)-BINAP]Ni(η2-NC-Ph) (0.010 mmol, 5% or 0.020 mmol, 10%); solvent: toluene (1.0 mL); ee was determined by chiral HPLC analysis.
