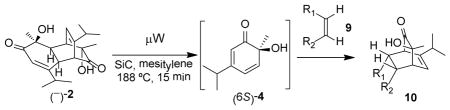
Table 2.
Retro-[4+2]/[4+2] reactions to afford bicyclo[2.2.2]-octenonesa
 | |||
|---|---|---|---|
| entry | dienophile (equiv) | cycloadductb | yieldc (%) |
| 1 |
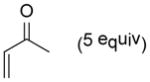 9a |
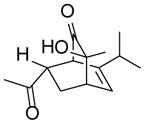 10a |
85 |
| 2 |
9b |
 10b |
99 |
| 3 |
9c |
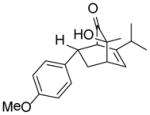 10cd |
98 |
| 4 |
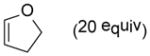 9d |
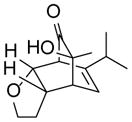 10d |
85 |
| 5 |
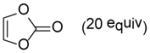 9e |
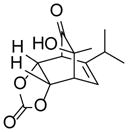 10ed |
76 |
| 6 |
9f |
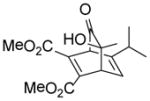 10f |
90 |
| 7 |
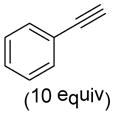 9g |
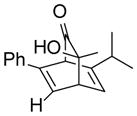 10gd |
80 |
Reaction conditions: dimer (−)-2, dienophile, SiC chip, mesitylene, μW, 180 °C, 15 min;
Single diastereomer isolated unless otherwise noted;
Isolated yield after column chromatography;
Approximately 9% of an inseparable minor product observed by 1H-NMR (ca. 10:1 isomeric ratio).
