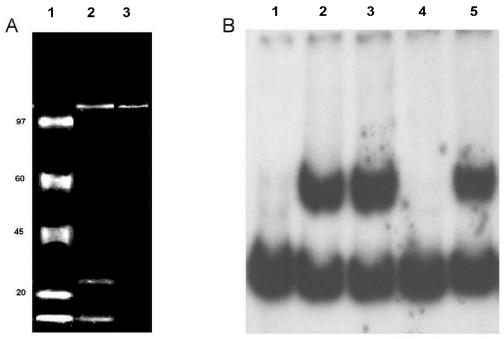
FIG. 7.
Purification of rep2037 by using concatemers of RBD. Concatemers of either wild-type RBD or the RBD double mutant were attached to magnetic particles (Dynal). (A) Purified proteins separated on an SDS-7% polyacrylamide gel. The gel was stained with Sypro Ruby and photographed with a Kodak digital system 120. Lane 1, molecular mass markers (from top to bottom, 97, 65, 45, 20, and <10 kDa); lane 2, purification of rep2037 from a crude ammonium sulfate fraction with RBD concatemer-labeled magnetic beads; lane 3, purification of rep2037 from the hydroxyapatite fraction with RBD concatemer-labeled magnetic beads. (B) Gel retardation analysis of rep2037 activity with RBD. Lane 1, 32P-labeled RBD probe alone; lane 2, 32P-labeled RBD probe plus rep2037 from hydroxyapatite fraction; lane 3, 32P-labeled RBD probe with supernatant of hydroxyapatite fraction previously extracted with the RBD double mutant concatemers coupled to magnetic beads; lane 4, 32P-labeled RBD probe with supernatant of hydroxyapatite fraction previously extracted with the wild-type RBD concatemers coupled to magnetic beads; lane 5, 32P-labeled RBD probe with eluate (panel A, lane 3) of wild-type RBD concatemers coupled to magnetic beads.