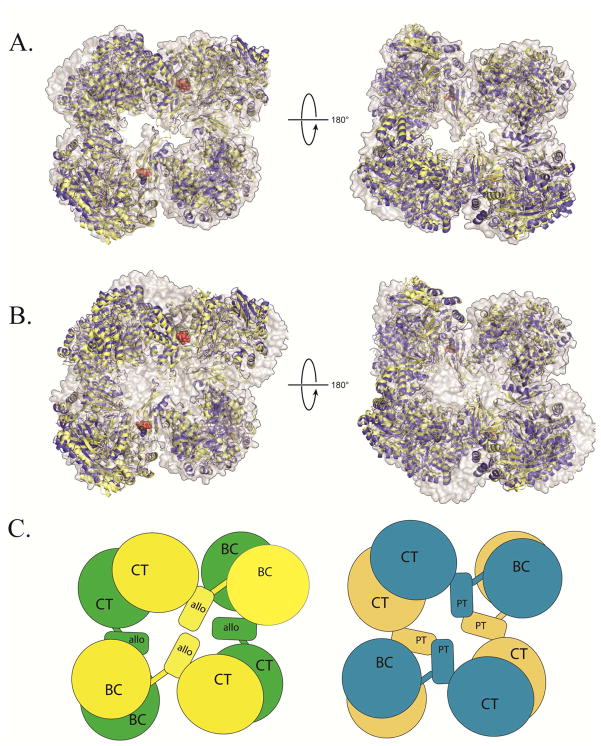
Figure 1. Structural superposition of PC X-ray crystal structures from R. etli and S. aureus.
(A) A structural superposition, viewed from the top (left) and bottom (right) face of T882A RePC + acetyl-CoA (yellow ribbons) and T882A RePC + L-aspartate (blue ribbons) with wild-type RePC + ethyl-CoA (2QF7; gray surface representation). For visual clarity, the alignment was centered on residues 450–1050 of chain A for all three structures, corresponding to the structurally rigid allosteric and CT domains. Alignment in this region results in a nearly perfect alignment at one corner of the tetramer and accentuates any remaining deviations in the structures. In the presence and absence of coenzyme-A analogues, all three structures of RePC are closely aligned. (B) A structural superposition, viewed from the top (left) and bottom (right) face of RePC T882A + acetyl-CoA (yellow ribbons) and RePC T882A + L-aspartate (blue ribbons) with wild-type SaPC + acetyl-CoA (3HO8; gray surface representation). The alignment was centered on residues 450–1050 of chain A for all three structures, corresponding to the structurally rigid allosteric and CT domains. The RePC structures do not align well with the SaPC + acetyl-CoA structure, regardless of whether acetyl-CoA is present in the RePC crystallization conditions. (C) A diagrammatic representation of the overall quaternary arrangement of RePC (left; top face = yellow; bottom face = green) and SaPC (right; top face = blue; bottom face = brown) emphasizing the absence of inter-subunit interactions between allosteric domains in RePC and the top-bottom subunit interactions between the central domains (PT = PC tetramerization) in SaPC.