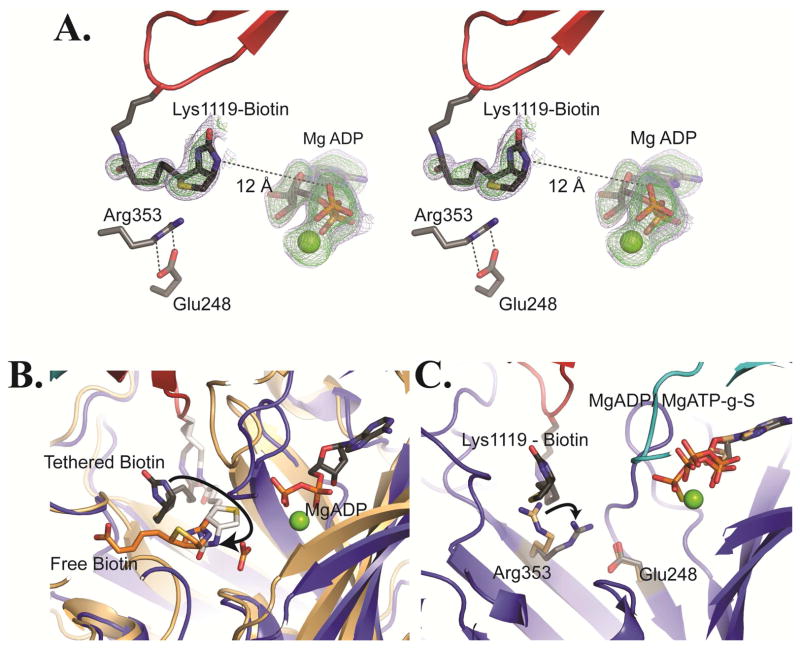
Figure 4. Tethered biotin in the BC domain of RePC.
(A) Stereoview of omit Fo – Fc electron density for tethered biotin and MgADP contoured at 2.0σ (blue) and 3.0σ (green) for chain B of T882A RePC. In this position, the N-1 of tethered biotin is positioned 12 Å from the β-phosphate of MgADP. The Arg353 and Glu248 side chains are illustrated as sticks. (B) The actual (gray) and modeled (white) position of tethered biotin in the BC domain active site of the T882A RePC crystal structure (tetramer 1, chain B). The structure is overlaid with that of E. coli BC (pdb id = 3G8C; colored in brown) co-crystallized with free biotin (orange) and bicarbonate. The modeled position demonstrates that tethered biotin can reach the active site and that the N-1 of tethered biotin can occupy a nearly identical position to the N-1 of free biotin in E. coli BC. Free biotin is oriented such that its valerate side-chain points away from the BCCP domain binding site. Consequently, tethered biotin cannot physically adopt the same binding orientation as free biotin. (C) A BC domain overlay of the bottom subunit of wild-typeRePC (chain B; pdb id = 2QF7) with the bottom subunit of tetramer 1 of T882A RePC (chain B). The wild-type RePC structure has ATP-γ-S bound in the active site and does not interact with the BCCP domain. Conversely, the RePC structure of T882A has MgADP bound in the active site and interacts with the BCCP domain. While the position of Glu248 remains unchanged between the two structures, Arg353 swings to interact with Glu248 in the T882A RePC structure. The side chain position of Arg353 in wild-type RePC is colored in brown while its position in the T882A RePC structure is colored in gray.