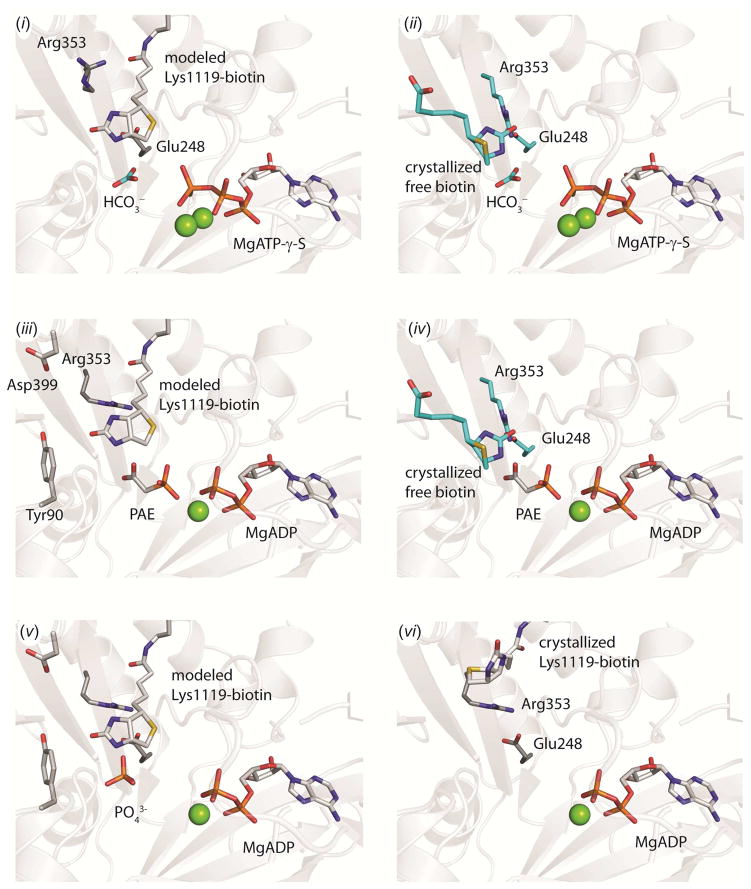
Figure 6. A structural model of catalysis in the BC domain. (i).
Ternary substrate complex with the modeled position of tethered biotin in the active site. The position of HCO3− is illustrated from an overlay with E. coli BC (pdb id = 3G8C, chain A) and MgATP-γ-S is illustrated from an overlay with the BC domain of wild-type RePC (pdb id = 2QF7, chain A). The open side-chain configuration of Arg353 and Glu248 from the structure of wild-type RePC (pdb id = 2QF7, chain A) is also shown. (ii) Ternary substrate complex with free biotin in the active site. The positions of free biotin, HCO3−, Arg353 and Glu248 are from an overlay with E. coli BC (pdb id = 3G8C, chain A) and the position of MgATP-γ-S is from an overlay with the BC domain of wild-type RePC (pdb id = 2QF7, chain A). (iii) Carboxyphosphate-bound intermediate state with modeled tethered biotin and phosphonoacetate (PAE) in the active site. Phosphonoacetate is displayed in a single conformation from an overlay with the BC domain of chain C in the T882A RePC structure. The position of MgADP, Tyr90, Asp399 and Arg353 side-chains are from an overlay with the BC domain of chain B in the T882A RePC structure. (iv) Carboxyphosphate-bound intermediate state with free biotin and phosphonoacetate in the active site. Phosphonoacetate is displayed in a single conformation from an overlay with the BC domain of chain C in the T882A RePC structure. The position of MgADP, free biotin, Arg353 and Glu248 side-chains are from an overlay with the structure of E. coli BC (pdb id = 3G8C, chain A). (v) Phosphate is positioned to serve as the general-base catalyst for the enolization of tethered biotin. The modeled position of tethered biotin in RePC is shown, along with the position of MgADP, Glu248, Asp399, Tyr90 and Arg353 from an overlay with the BC domain of chain B in the T882A RePC structure. (vi) Product-bound state with MgADP in the active site and tethered biotin excluded by the closed conformation of Arg353. The position of all residues and ligands are from the crystal structure of T882A RePC, chain B.