Abstract
Thickening of the intimal layer of arteries characterized by expression of smooth muscle α-actin (SMαA), collagen deposition, and inflammation is an important pathophysiological change with aging assumed to be mediated by smooth muscle cells migrating from the medial layer. We tested the novel hypothesis that these characteristics could also reflect an endothelial-mesenchymal (smooth muscle-like) transition (EnMT). Late (‘old’) compared with early (‘young’) passage (45.0 ± 1.2 vs. 27.1 ± 0.5 population doublings) human aortic endothelial cells demonstrated greater smooth muscle (spindle) morphological changes, expression of SMαA and collagen I, nuclear factor-κB activation, and transforming growth factor-β (TGF-β) (all p < 0.05). Based on increases in SMαA, stimulation with the proinflammatory cytokine tumor necrosis factor-α, but not with TGF-β, induced EnMT in early passage cells similar to that observed in late passage cells. Here, we present the first evidence for EnMT induced in a model of endothelial cell aging and provide support for proinflammatory signaling in mediating this phenotypic change.
Key Words: Senescence, Tumor necrosis factor-α, Arterial stiffness, Intimal medial thickness
Introduction
Cardiovascular diseases (CVD) remain the leading cause of death in modern societies. Advancing age is the major risk factor for CVD and this is attributable primarily to pathophysiological changes in arteries [1]. These include structural remodeling featuring thickening of the intimal (inner) layer, increased expression of smooth muscle α-actin (SMαA), increased collagen synthesis/deposition, and inflammation linked to increased nuclear factor-κB (NFκB) and tumor necrosis factor-α (TNF-α) signaling [1,2,3,4]. The mechanisms involved are incompletely understood, but it has been assumed that these changes are caused by the actions of resident vascular smooth muscle cells that proliferate and migrate from the medial (middle) layer to the intimal layer [3]. However, recent work indicates that in certain disease states such as pulmonary hypertension and fibrotic kidney disease, endothelial cells may transition to a ‘mesenchymal’ (smooth muscle-like) phenotype characterized by morphological changes, SMαA expression, and increased secretion of collagen [5,6,7]. Inflammation has been hypothesized as a potential trigger for this transition and increases in transforming growth factor-β (TGF-β) as a possible subsequent but early initiating factor [5,7]. It is unknown if this type of phenotypic transition can occur in a setting of cellular aging and, if so, whether inflammatory signaling or TGF-β are involved.
Here, we test the novel hypothesis that human endothelial cell aging results in an endothelial-mesenchymal transition (EnMT). To do so, we used a model of arterial endothelial cell aging in which human aortic endothelial cells are studied in early (‘young’) and late (‘old’) stages of replication [8,9]. We also sought to gain insight into the possible roles of increased proinflammatory signaling and TGF-β in mediating EnMT in this model.
Methods
Human aortic endothelial cells were purchased from a commercial vendor (Lonza, Walkersville, Md., USA). These cells were characterized by positive staining for acetylated LDL and von Willebrand and negative staining for SMαA according to the manufacturer's product sheet. In the initial culture (baseline), these cells did not express the smooth muscle marker, smooth muscle myosin heavy chain. All experiments were performed on primary cells from two different donors. Cells were obtained from a 65-year-old female and a 41-year-old male. Cells were grown in endothelial growth medium-2 (EGM-2) that was supplemented with growth factors, antibiotics, and 2% fetal bovine serum (EGM-2 SingleQuot; Lonza). Cells were passaged at ∼80% confluence and seeded at a density of 2,500–5,000 cells/cm2. All cells were studied at a confluence of ∼75–80%.
Population doubling levels (PDL) were calculated by taking the log2 of the number of cells (living and dead) counted at a passage divided by the initial number of cells seeded on the plate [PDL = log2 (number of cells at passage/number of initial cells)]. Cumulative PDL were calculated by summing the PDL from the current passage to the PDL of the previous passage (cumulative PDL = PDL of the current passage + PDL of the previous passage).
Whole cell preparation Western blotting was performed as previously described [10]. Briefly, early and late passage cells with or without 24-hour treatment with TGF-β (1–100 ng/ml; R&D Systems, Minneapolis, Minn., USA) or TNF-α (1–100 ng/ml; R&D Systems) were lysed in ice-cold RIPA buffer. Equal amounts of protein were loaded into a polyacrylamide gel and transferred to a nitrocellulose membrane. Primary antibodies for type I collagen (1:2,000; Millipore, Billerica, Mass., USA), SMαA (1:10,000; Epitomics, Inc., Burlingame, Calif., USA), collagen IV (1:500; Millipore), CD31, an endothelial cell marker (1:500; Abcam, Cambridge, Mass., USA), and glyceraldehyde phosphate dehydrogenase (GAPDH) (1:2,000; Cell Signaling Technology, Inc., Danvers, Mass., USA) were incubated overnight at 4°C. A secondary HRP-conjugated antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa., USA) was incubated at room temperature for 1 h. Membranes were developed using ECL (Pierce/Thermo Scientific) and visualized using a digital acquisition system (ChemiDoc-It; UVP, Inc., Upland, Calif., USA). Collagen and SMαA bands are expressed relative to GAPDH and normalized to the control group.
Senescence-associated β-galactosidase staining was performed on early passage cells, early passage cells treated with TGF-β (10 ng/ml), early passage cells treated with TNF-α (10 ng/ml), and late passage cells. Cells were grown on 4-well culture slides and fixed in 2% formaldehyde/0.2% glutaraldehyde solution for 5 min. Cells were washed with PBS and treated for 24 h at 37°C with β-galactosidase staining solution [5 mM potassium ferricyanide, 2 mM magnesium chloride, 150 mM sodium chloride, 30 mM citric acid, 5 mM potassium ferrocyanide, 12 mM sodium phosphate, pH 6.0, containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal; Sigma)]. Slides were washed with PBS, cover slipped, and analyzed under a light microscope.
Data are presented as means ± SEM. Statistical analysis was performed with PASW 18.0 software (PASW, Inc., Chicago, Ill., USA). A 2-tailed t test was used to compare the differences between early and late passage cells. A one-way ANOVA was used for TGF-β and TNF-α experiments with post hoc analyses where appropriate. p < 0.05 was considered statistically significant.
Results
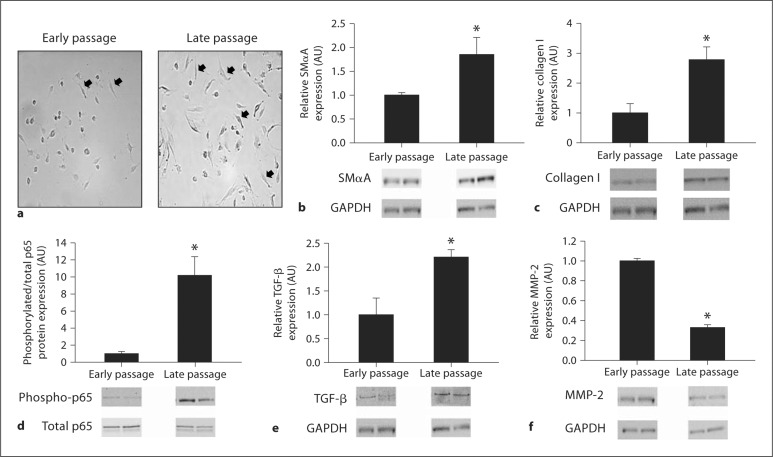
Population doublings were greater in late compared with early passage human aortic endothelial cells (45.0 ± 1.2 vs. 27.1 ± 0.5, p < 0.05). Late passage cells demonstrated increased spindle (smooth muscle-like) morphological changes (fig. 1a), an 85% greater expression of SMαA (1.85 ± 0.36 vs. 1.00 ± 0.05, p < 0.05) (fig. 1b), an ∼180% greater collagen I expression (2.78 ± 0.43 vs. 1.00 ± 0.31, p < 0.05) (fig. 1c), a 10-fold greater expression of the phosphorylated-to-total p65 subunit of NFκB (10.17 ± 2.20 vs. 1.00 ± 0.26, p < 0.05) (fig. 1d), a 120% greater expression of TGF-β (2.21 ± 0.16 vs. 1.00 ± 0.35, p < 0.05) (fig. 1e), and a 67% reduction in MMP-2 expression (0.33 ± 0.03 vs. 1.00 ± 0.03, p < 0.05) (fig. 1f) compared with early passage cells. CD31 (1.26 ± 0.29 vs. 1.00 ± 0.19) and collagen IV (1.09 ± 0.19 vs. 1.00 ± 0.20) expressions were not different between late and early passage cells (data not shown).
Fig. 1.
Markers of EnMT in early and late passage human aortic endothelial cells. Spindle morphology (a), SMαA (b), collagen type I protein (c), phosphorylated-to-total p65 subunit of NFκB (d), TGF-β (e), and MMP-2 (f) in early and late passage human aortic endothelial cells. Values are means ± SEM. ∗ p < 0.05 versus early passage; black arrows denote spindle morphology.
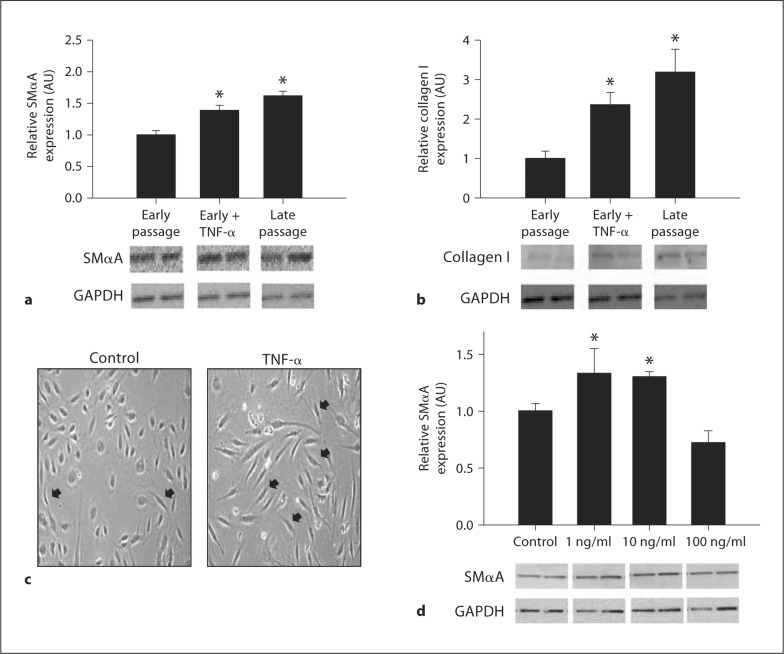
To determine if inflammation can induce this EnMT, early passage cells were first incubated with 10 ng/ml of TNF-α for 24 h. Incubation with TNF-α increased expression of SMαA (1.39 ± 0.08 to 1.62 ± 0.07, p < 0.05) (fig. 2a) and collagen I (2.36 ± 0.31 to 3.19 ± 0.58, p < 0.05) (fig. 2b) to levels not significantly different from those of late passage cells while inducing a late passage spindle phenotype (fig. 2c). Twenty-four-hour dose-response experiments revealed increases in SMαA with 1 and 10 ng/ml (both p < 0.05), but not 100 ng/ml concentrations of TNF-α (fig. 2d).
Fig. 2.
TNF-α stimulation of EnMT in human aortic endothelial cells. SMαA (a) and collagen type I protein (b) expressions in early passage, early passage treated with TNF-α (10 ng/ml), and late passage human aortic endothelial cells. c Spindle morphology in early passage and early passage treated with TNF-α (10 ng/ml) human aortic endothelial cells. d SMαA in response to increasing doses of TNF-α. Values are means ± SEM. ∗ p < 0.05 versus early passage, control; black arrows denote spindle morphology.
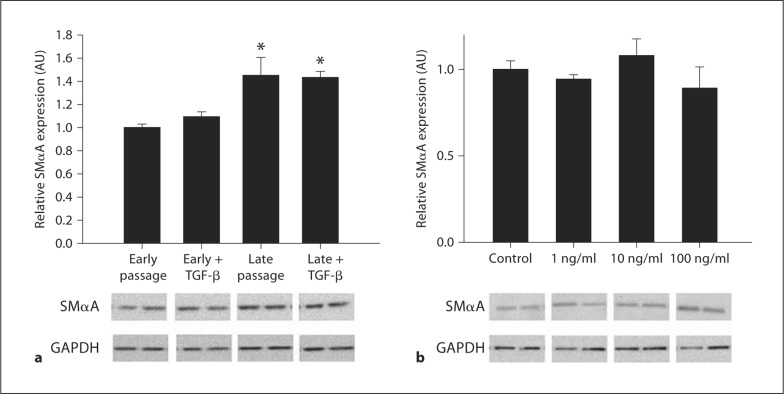
To determine if TGF-β can induce the EnMT, early passage cells were first incubated with 10 ng/ml of TGF-β for 24 h. In contrast to TNF-α, incubation with 10 ng/ml of TGF-β had no effect on SMαA in young passage cells or on late passage cells (fig. 3a). Further dose-response experiments revealed no effects of TGF-β on SMαA at doses from 1 to 100 ng/ml (fig. 3b).
Fig. 3.
Effects of TGF-β in human aortic endothelial cells. a TGF-β (10 ng/ml) on SMαA expression in early and late passage human aortic endothelial cells. b SMαA in response to increasing doses of TGF-β. Values are means ± SEM. ∗ p < 0.05 versus early passage, early passage treated with TGF-β.
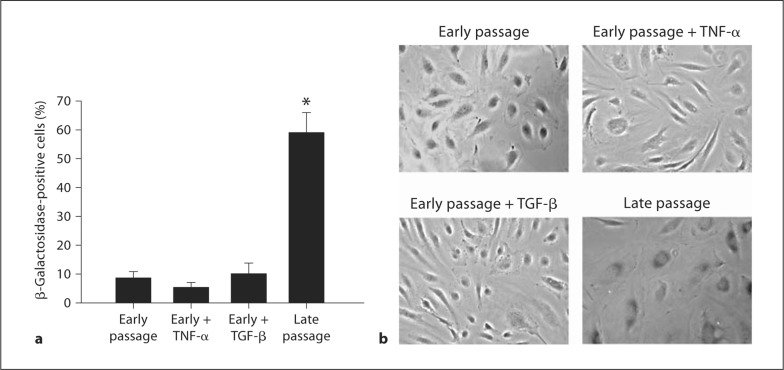
Late passage cells demonstrated markedly increased staining for β-galactosidase compared with early passage cells (p < 0.05) (fig. 4). Incubation with TNF-α or with TGF-β (both 10 ng/ml) did not increase β-galacosidase staining in early passage cells compared with early passage cells studied under control conditions (fig. 4).
Fig. 4.
Senescence in TNF-α, TGF-β, and late passage human aortic endothelial cells. a Percent of positive β-galactosidase-stained cells. b Representative images in early passage, early passage treated with TNF-α (10 ng/ml), early passage treated with TGF-β (10 ng/ml), and late passage human aortic endothelial cells. Values are means ± SEM. ∗ p < 0.05 versus all.
Discussion
The present results show for the first time that EnMT can occur in a model of cellular aging. This is supported by the observation that several key features of EnMT including morphological changes and increased expression of SMαA and collagen I were greater in late compared with early passage human aortic endothelial cells, with evidence for activation of the proinflammatory nuclear transcription factor NFκB and the cytokine TGF-β despite reductions in MMP-2. We also observed a marked increase in β-galactosidase staining, a marker of senescence, in late compared with early passage cells. Previous reports indicate that some vascular disease states are associated with EnMT [5,6,7]. The present findings extend this earlier work by showing that a model of primary human endothelial cell aging can also induce EnMT.
Our results also provide evidence for a role of inflammation in inducing EnMT with cellular aging. NFκB activation and increased expression of TNF-α are observed in vascular endothelial cells from older healthy adult humans [11,12] and in arteries of old rodents [2] and are related to vascular endothelial dysfunction [11,12,13]. Here, we show that this proinflammatory state can trigger EnMT in early passage human arterial endothelial cells similar to the phenotype observed in late passage cells. In contrast, the present results do not support an obvious role for TGF-β, which has been proposed as a possible initiating factor in EnMT associated with vascular disease [5]. Neither TNF-α nor TGF-β induced an increase in β-galactosidase staining in early passage cells, suggesting that development of EnMT may precede senescence in this model.
Intimal thickening featuring increased collagen deposition and inflammation is one of several key pathophysiological changes that occur in arteries with aging that increase the risk of CVD. As such, the present findings have important implications for the cellular and molecular mechanisms by which aging adversely effects arteries to increase their susceptibility to the development of clinical vascular diseases.
Acknowledgements
This study was supported by NIH AG013038 and HL007822. The authors thank Thomas LaRocca for his technical assistance.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. 1. Aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33:116–123. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Zhang J, Jiang L-Q, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 5.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 6.O'Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Heart Circ Physiol. 2007;292:H285–H294. doi: 10.1152/ajpheart.00560.2006. [DOI] [PubMed] [Google Scholar]
- 7.Vyas-Read S, Shaul PW, Yuhanna IS, Willis BC. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L212–L221. doi: 10.1152/ajplung.00475.2006. [DOI] [PubMed] [Google Scholar]
- 8.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 10.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress With aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappa B. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 12.Donato AJ, Black AD, Jablonski KL, Gano LG, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]