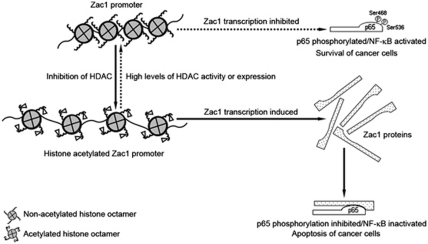
Figure 8.
Summary by schematic illustration. The histone of the Zac1 promoter in untreated EC cells was hypo-acetylated, which repressed Zac1 gene transcription. HDAC inhibitors induced histone acetylation and increased Zac1 expression. Zac1 protein bound to the NF-κB p65 subunit and inhibited its phosphorylation at Ser468 and Ser536, leading to suppression of NF-κB activity and induction of cell apoptosis. The reverse process, as indicated by a dashed arrow, shows a potential pathway that maintains NF-κB activity and is important for cancer cell survival. High levels of HDAC or HDAC activity maintained the Zac1 promoter in a hypo-acetylated state and thus suppressed Zac1 expression, which accounted for the relatively high phosphorylation levels at Ser468 and Ser536 residues of p65 protein and the relatively high NF-κB activity