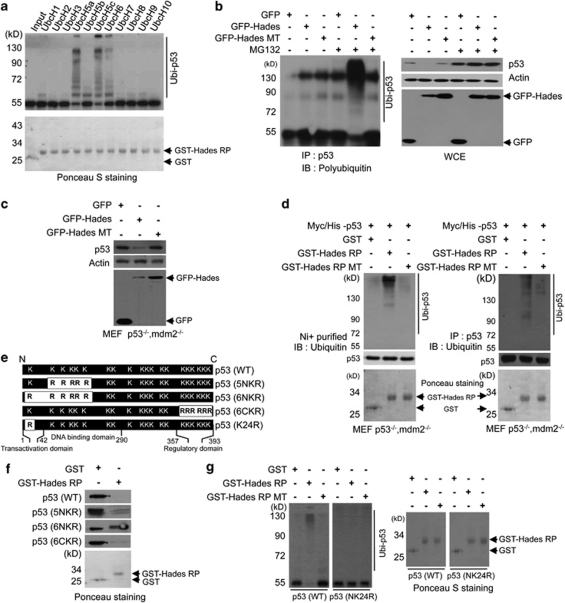
Figure 3.
Hades promotes polyubiquitination of p53. (a) Hades ubiquitinates p53 in vitro. We incubated in vitro translated, 35S-labelled p53 with GST-tagged Hades RING finger peptide (aa 271–351) (GST-Hades RP), ATP, His-ubiquitin, E1, and E2 enzymes. Reaction products were analyzed by autoradiography. GST and GST-Hades RP were measured as controls. (b) Hades ubiquitinates p53 in vivo. p53 null H1299 cells were cotransfected with plasmids for p53 (2 μg) and GFP-Hades or GFP-Hades MT (4 μg). At 24 h after transfection, cells were incubated with or without MG132 (10 μM) for 4 h. Anti-p53 immunoprecipitates were analyzed using anti-polyubiquitin antibody. GFP-Hades and p53 protein levels in whole-cell extracts were measured. (c) Mdm2 is dispensable for Hades-mediated p53 degradation. MEF p53−/− mdm2−/− cells were transfected with 1 μg of indicated plasmid. After 24 h, GFP and p53 levels were analyzed. (d) Hades ubiquitinates p53 in MEF p53−/−mdm2−/− cells. MEF p53−/−mdm2−/− cells were transfected with plasmid for His-p53. At 24 h after transfection, cells were harvested and the lysates were incubated for 2 h with GST, GST-Hades RP, or GST-Hades RP MT. p53 was pulled down with Ni+-conjugated beads (left) or immunoprecipitated using anti-p53 antibody (right). Precipitated proteins were analyzed using anti-ubiquitin antibody. Ponceau S staining of GST, GST-Hades RP, and GST-Hades RP MT were measured as loading controls. (e) Schematic of p53 lysine mutants. 5NKR was mutated at five N-terminal lysine residues (aa 101, 120, 132, 139, and 164), 6NKR at six N-terminal lysine residues (aa 24, 101, 120, 132, 139, and 164), 6CKR at six C-terminal lysine residues (aa 370, 372, 373, 381, 382, and 386), and K24R at one N-terminal lysine residue (aa 24). (f) Hades ubiquitinates p53 at the N-terminal residue, lysine 24. In vitro ubiquitination was assessed using in vitro translated p53 and GST-Hades RP. Naïve proteins in reaction product were analyzed by immunoblotting using anti-p53 antibody. GST and GST-Hades RP were measured as controls. (g) Hades cannot ubiquitinate the p53 NK24R mutant. In vitro ubiquitination was assessed with in vitro translated, 35S-labelled WT or N24KR mutant p53 and GST, GST-Hades RP, or GST-Hades RP MT. Reaction products were separated and analyzed by autoradiography. GST, GST-Hades RP, and RP mutant were measured as controls