Figure 6.
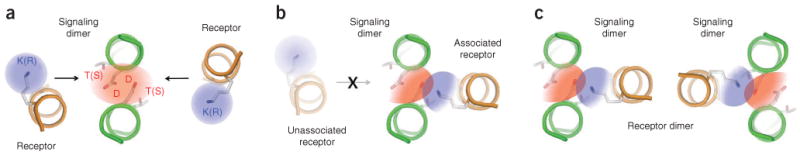
Proposed model for intramembrane immunoreceptor complex assembly. (a) Dimeric signaling modules (CD3, DAP10 or DAP12; green coils) exist as metastable intermediates in the endoplasmic reticulum membrane, whereas newly synthesized receptor subunits (orange coils) are either incorporated into complexes or rapidly degraded4,27. The electrostatic network in the signaling dimer (red shading) shows a symmetrical electron distribution and may have multiple opportunities to assemble with basic residues (blue shading) from receptor subunits. K(R), lysine or arginine; T(S), threonine or serine, (b) Once a receptor subunit has associated with an available binding site, an asymmetric redistribution of electronegativity in the network (color gradients) may render the opposite side of the signaling module unable to bind a second receptor. This trimeric assembly therefore represents the fundamental structural unit that organizes immunoreceptor complexes, (c) In a step that is either subsequent to or simultaneous and cooperative with that in b, receptors that form dimers (such as TCR, NKG2D and the mouse Ly49 family) combine two or more trimeric units to form the final complex. The relative orientation of the individual trimeric units cannot be determined from structural or biochemical data available at present.
