Table 1.
Antibacterial activities of compounds 1, 2, 10, 15, 16 and 21–27.a
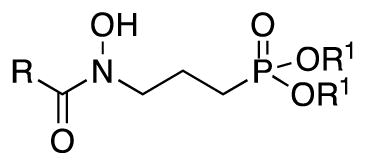
| Compound | R | R1 | Gram (+)
|
Gram (−)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. anthracis | E. faecalis | S. aureus (MSSA) | S. aureus (MRSA) | M. tuberculosis (H37Rv) | Acineto-bacter | E. coli k12 | E. coli tolc | |||
| Fosmidomycin (1) | H | H/Nab | 0.78 | >200 | >200 | 200 | >500 | 100 | 12.5 | 6.25 |
| FR900098 (2) | CH3 | H/Nab | 50 | >200 | >200 | 50 | >500 | 50 | 200 | 12.5 |
| 10 | H | Et | >200 | >200 | >200 | >200 | 400 | >200 | >200 | >200 |
| 15 | CH3 | Et | 200 | >200 | >200 | 200 | 200–400 | >200 | >200 | >200 |
| 16 | CH3 | iPr | >200 | >200 | >200 | >200 | 400 | >200 | >200 | >200 |
| 21 | CH3 |
|
NDc | ND | ND | ND | 100 | ND | ND | ND |
| 22 | CH3 |
|
>200 | >200 | >200 | >200 | 400 | >200 | >200 | 200 |
| 23 | CH3 |
|
>200 | >200 | 100 | 25 | 400 | >200 | >200 | >200 |
| 24 | CH3 |
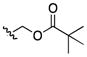
|
200 | >200 | 100 | 50 | 50–100 | >200 | >200 | 100 |
| 25 | CH3 |
|
200 | >200 | 100 | 50 | 400 | >200 | >200 | >200 |
| 26 | CH3 |
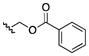
|
100 | >200 | >200 | >200 | 25–100 | 200 | >200 | 50 |
| 27 | CH3 |
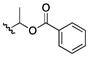
|
50 | >200 | >200 | >200 | 100–200 | 100 | 50 | 50 |
Minimum Inhibitory Concentrations (MIC) in μg/mL.
Compounds 1 and 2 used as the monosodium salts.
ND = not determined.
